| Curved Film Frost, Part 1: On the General Causes » |
A greenhouse effect demonstration that you can feel with your hand
Probably most people consider the greenhouse effect as an important, yet subtle, effect on our climate. I suspect though that very few consider it the major effect on our daily weather, amenable to direct sensation, that it really is. Perhaps this lack of appreciation can be partly alleviated with a simple and quick demonstration. Below is my attempt at such a demonstration. This demo focuses on a key aspect of the greenhouse effect. It requires only items typically found in a kitchen yet allows one to directly feel the warming. An added bonus is that the specific phenomenon illustrated also helps explains other phenomena we witness in daily life. Below are all the things you need to do the demo. (click on any pic to enlarge)
What does the greenhouse have to do with snow?
But first, as this is a snow blog, what is the connection to snow? The growth of snow crystals is, except for their being in free-fall, almost exactly the same as the growth of hoar frost on the ground. The hoar frost on the ground occurs when the local greenhouse effect during the evening and night is relatively weak. That is, the local atmosphere above is cloud-free and relatively dry. (Of course, the air at the ground needs to be relatively cold and humid as well.) This connection illustrates one way we directly sense variations in the greenhouse effect through our weather. More on this later.
A key aspect of the greenhouse effect
The greenhouse effect involves several connected concepts, or aspects, a fact that makes the complete effect much more challenging to understand than is commonly appreciated. Nevertheless, we can readily understand one of its crucial aspects: emitted infrared radiation (IR) from the atmosphere directly warms us on the ground. This IR radiation from above, also known as “back radiation”, is not small: indeed, averaged globally and annually, the radiated energy we receive from atmospheric IR is roughly twice that which comes from the sun. (Why do we not notice such a large effect? Because, unlike sunshine, it never stops.) Unfortunately, this is not how the effect is usually presented in the popular literature. For more details about this issue and other greenhouse misconceptions, I urge you to read Alistair Fraser’s excellent and amusing “Bad Greenhouse” page (1), but here I focus on a simple demo.
Simple demos of the effect
Most demos of the greenhouse instead focus on atmospheric absorption of infrared by greenhouse gases. Although absorption of IR is part of the greenhouse effect, to understand how we are warmed at the ground, one needs additional concepts to complete the explanation, concepts that are usually left out of the discussion. More to the point for this blog entry, of the demos that I’ve seen, all of them misrepresent the atmospheric greenhouse. Specifically, instead of illustrating a heating process in the atmosphere, such demos instead illustrate the heating process in a gardener’s greenhouse. This is the wrong effect. If you are skeptical of this claim, consider the highlighted text in the following study (2):
A similar study was published a few years later in the European Journal of Physics. But it is not just the physicists who think the popular demos are wrong, here is a similar study from chemists (3):
The title of that study should also make their point clear. Let me emphasize here that the demos that these studies criticize are actually the best available ones, for example the one use by Bill Nye, “the science guy”. A quick survey of other demos readily found on the Internet (refs 4-7) takes me to ones that are even less relevant to our atmosphere (sadly, even ones suggested by NASA). It is also unfortunate that even the above two studies use terms "climate change" and "global warming" in their titles, even though they are really about the greenhouse effect. These are distinct concepts, the former being about an increase in the latter. We are interested here only in the greenhouse effect.
As pictured in the first image above, all you need for this demo is a stove (or hotplate), a baking pan (if using the stove), oven mitts, new aluminum foil, a baking sheet (i.e., oven-safe paper), and a few teaspoons of vegetable oil. For extra safety, use protective eyewear. Total time needed once you have the pieces set out, is only about 15 minutes. No thermometer is needed because you can feel the warmth with your hand.
1) Set the baking pan on the stove burner, centered if possible.
2) Put a little vegetable oil in the center and press on a section of aluminum foil to cover the base of the pan as completely as possible (can extend over the edges). Use an oven mitt to press the foil flush against the pan.
3) Turn the burner on to “medium” and wait about 10 minutes for it to heat up.
4) While waiting for the burner to warm up, tear off a piece of baking sheet large enough to nearly fill the area of the pan. Set it aside.
Once the pan has warmed up (carefully touch an edge of the pan to check), the experiment begins.
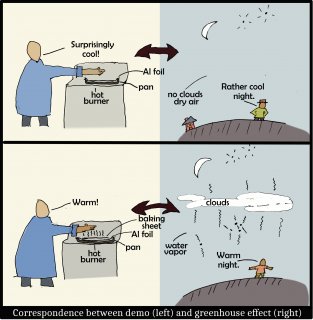
A) Put your hand a few inches above the center of the aluminum foil. Does it feel warm? WARNING: NEVER TOUCH THE FOIL SURFACE! IT IS VERY HOT!
B) Now put a little vegetable oil on the aluminum foil and carefully drop the baking sheet on the foil, trying to set it down without touching the hot surface so it covers nearly all of the foil. With the oven mitt on your hand, quickly press the baking sheet down so there are no air gaps between it and the foil (this is the purpose of the oil).
C) Now, repeat A). Does it feel noticeably warmer? (Before the baking sheet starts to burn, please use the mitts to move the pan off the burner and turn off the burner.)
It seems weird that you can put a cool object (the baking sheet) between your hand and a hot surface to actually warm your hand. Nevertheless, your hand is warmer because the baking sheet is an effective radiator of IR, whereas the Al foil is a very poor radiator of IR. (This is why hot items are wrapped in foil to stay warm.) For this reason, the adding of Al foil to the pan likely made the pan even hotter--it reduced the capability of the pan to cool. With the oil between the pan and foil, and between the foil and baking sheet, all three were put in good thermal contact, exchanging heat via conduction and thus having essentially the same temperature. However, the the top layer (i.e., the foil in A, the baking sheet in C) cools by conducting to the air above, so the top surface will actually be slightly cooler than the pan. However, in general, the temperature of a hot object mostly cools by emitting IR, unless it is covered by a poor IR emitter like Al foil.
In this experiment, it is too easy to be fooled into thinking the foil is not hot because it does not feel hot a few inches away. However, if you touch it with bare finger, your finger will get a burn due to conduction.
The following sketch shows why adding a sheet on top is analogous to the adding of greenhouse gases or clouds to the atmosphere.
In brief, the baking sheet, like greenhouse gases (including water vapor) and clouds, are very effective at radiating IR. The air in the atmosphere may be warm, yet is not radiating until greenhouse gases or clouds are present. So, on clear, dry nights, we are cooler simply because there is very little above us that radiates IR down.
This same phenomenon is often presented in a different way that is, unfortunately, misleading: Popular accounts often say that the clouds and greenhouse gases are “holding in the heat” or “trapping the heat”, and thus (as they say) without the clouds (or greenhouse gases), the ground can now radiate to space and cool down. This idea is misleading because a) this radiating of the ground is always occurring, day and night, cloudy and clear. By radiating, it is always cooling the ground, indeed, even more so when it is warmer than when it is cooler. Moreover, b) when this IR going up from the ground reaches the clouds, it does not “reflect” back down, but instead will be absorbed. Some is also absorbed by greenhouse gases, and some of it continues to outer space.
Finally, the heating effect we feel on the ground has nothing to do with any IR that is absorbed by the clouds. The clouds, as well as the greenhouse gases, radiate IR merely because they have a non-zero temperature (i.e., are warmer than outer space). How the air gets that temperature is immaterial. In general, most of the heating of the air is due to convection, with some of it from absorption of IR. My suggestion to avoid being mislead and confused: do your best to forget anything about “trapping” heat and consider the radiating aspects of the atmosphere as I have emphasized here.
Other than the greenhouse effect varying with cloud cover from one day to another, you can experience differences in the greenhouse effect by travelling from a desert to the tropics. The greenhouse effect is much larger in the tropics due to the greater cloudiness and humidity there. In the tropics, the day-night temperature difference may be only a few degrees. In contrast, in the clear, dry desert, the day-night temperature difference is often tens of degrees. You see, it is all about the amount of radiating gas, vapor, and clouds above.
One last example of why thinking of the emission of IR, not the absorption, can be more illuminating. You might have heard that with the average warming we have experienced the past few decades due to increasing greenhouse gases, the upper atmosphere is actually cooling. Why is this? How can it be cooling? The upper atmosphere has also received more greenhouse gases from below, but does not receive much heating from the ground below via convection, as does the lower atmosphere. So here in the upper atmosphere, having more greenhouse gases mainly means that the air here radiates more. As it radiates more, it cools more.
Finally, this demo helps one to understand other everyday phenomena. You might have wondered why, for example, insulation for hot-water heaters, hot-water pipes, and thermoses tend to be shiny and metallic. The answer is that, like the Al foil in the demo, such a surface is a poor emitter of IR, so the enclosed items do not cool as rapidly. Another application is also inside the home. On cold days, you might notice that rooms with outside walls, windows, and floor may feel cooler in winter than interior rooms, despite the air temperature being the same. The reason is that the interior walls and floors radiate more IR than colder outside walls, windows, and floors, and so you feel warmer because you actually are warmer. This is just like being warmer outside on a night with cloud cover.
The picture here of the greenhouse effect is very simplified, but I hope the demo nevertheless helps to convey the widespread importance of IR emission, both in the atmosphere and at home.
--JN
References
1) Bad Greenhouse: https://personal.ems.psu.edu/~fraser/Bad/BadGreenhouse.html
2) Paper in 2010 American Journal of Physics: https://rtobin.phy.tufts.edu/Wagoner%20AJP%202010.pdf See also the paper in the European Journal of Physics: https://iopscience.iop.org/article/10.1088/0143-0807/35/2/025016
3) The similarly critical paper in 2019 Journal of Chemical Education: https://pubs.acs.org/doi/pdf/10.1021/acs.jchemed.8b01057
4) And now, a sampling of bad demos. Please do not use them in a classroom. https://www.steampoweredfamily.com/the-greenhouse-effect-experiment/ (mainly a diversion about a chemical reaction, otherwise seems to be about the florist’s or gardener’s type of greenhouse, not our atmosphere)
5) Like the above, it is about the gardener’s greenhouse: https://www.sciencefun.org/kidszone/experiments/the-greenhouse-effect-weather-science-experiment/
6) More scientific aspects in this one, yet it is still about the gardener’s greenhouse: https://edu.rsc.org/experiments/modelling-the-greenhouse-effect/1543.article
7) Sadly, even NASA has the gardener’s greenhouse demo in mind here (4th “topic idea” down the list): https://climatekids.nasa.gov/science-fair/