| « A greenhouse effect demonstration that you can feel with your hand | Avalanches, Part 2: Effect of Debris » |
Curved Film Frost, Part 1: On the General Causes
As I've seen it, the three most common ways to depict coldness in art and culture are the snow crystal, the icicle, and the curving frost on windows.
All of these have seen relatively little study, but without question the last has received the least. Indeed, I know of only three studies dedicated mainly to curving frost, and all three are over 50 years old.
It is perhaps little known that in terms of being observable to the typical observer, the third would likely be far more common. An odd thing to say? Well, people may say that they see it far less now due to the common use of central heating, or some might say that global warming is making it harder to form, but in these arguments they are wrong. I think the opposite: it is in fact far easier to see it now than maybe anytime in the past due to the ubiquitous presence of cars parked outdoors.
One curious thing about curved frost is its defining feature, the curviness. This feature contrasts with the straightness (and regularity) of snow crystals. What is the cause of the curviness in the former, as contrasts with the latter?
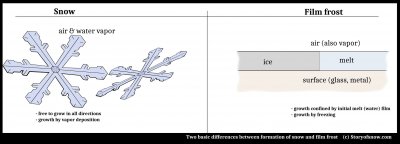
One key difference between the two cases is that the snow crystal grows from the vapor, whereas the curved frost grows in a thin film of water. Hence, the name I have been using for the latter is "film frost". So, snow grows by vapor deposition, film frost by freezing of melt.
Another key difference is that the snow crystal grows freely in space, whereas film frost grows along a surface. That is, the film frost is confined by two interfaces.
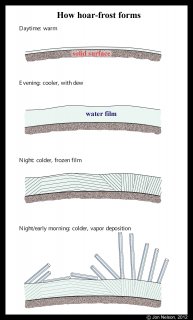
The main pattern of ice, the various curves and shapes, arise from the freezing film. However, after a section of film freezes, it usually grows hoar frost. Indeed, it is thought that all hoar frost starts this way, as I sketched in my post from 2012 (1).
However, with many cases of hoar frost, the surface is too bumpy and the hoar too long to discern the underlying film-frost pattern (e.g., on leaves and grass). We tend to discern the curvy patterns mainly on large, smooth surfaces.
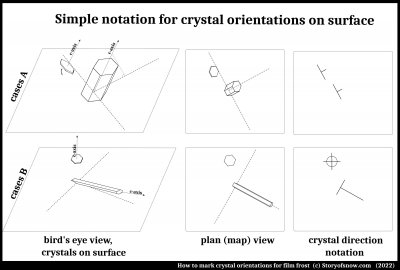
About the curving pattern, an early study (2) found that a) both the pattern curved and b) the crystal structure bent. The bending of the crystal structure has important implications because the structure tends to bend itself into a particular orientation. This orientation is such that the main symmetry axis, the "c-axis" is pointing straight up off the surface. Here is how the orientation aspect works:
In the top row above, both crystals have the same orientation relative to the surface (the area at bottom). The c-axis is like the axle around which the hexagonal plate or cylinder could spin, pointing up and partly north-eastern, if the map view has north in the standard direction. The direction could be specified with two angles, but instead the simple two bars at right are used. They are the same for both crystals in cases A because both have the same direction of c-axis.
In the bottom row, cases B, the two crystals have different c-axis directions, the one at top pointing straight out of the surface (i.e., "normal" to the surface), the other mostly pointing south-east, just a little above the surface (like pointing toward the rising sun in winter, if one is at a high northern latitude). For these cases, the symbols are also different as shown in the bottom right panel. Notice, as in the top row, that the length of the main "stem" of the "T" in the notation has nothing to do with the size or shape of the crystal, but depends only on its c-axis direction.
Given this notation, it is easier to show how the crystal structure bends as the crystal pattern itself curves. We will later, in part 2, show how one can see this directly in patterns such as the one shown at the top of this blog posting. Study (2) found that each "branch" of film-frost ice on a surface would curve and bend as sketched below.
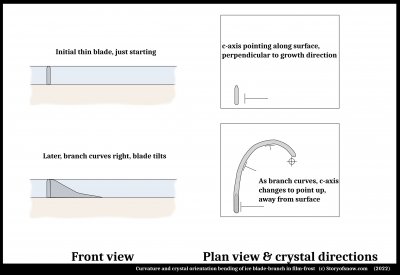
The basic structure of ice growing into supercooled melt, as the case here, is a thin blade as sketched at top left. The exact shape of the blade is not known, but you can readily see this basic thin-blade shape if you come across ice freezing across a puddle or pond. Carefully reach in, preferably using some sort of extension such as a twigs or chopsticks, and pull the ice out.
The crystal orientation is known to be very nearly set such that the c-axis is normal (perpendicular) to the direction of fast growth. The branch thickens at a much slower rate than it extends. Later, as sketched at bottom left, the branch curves right as the crystal's c-axis tilts up, eventually pointing straight up out of the surface. Once in this orientation, the crystal spreads out broadly and does not change orientation.
The question about the exact shape of this thin blade is a good one, and one that may be difficult to determine experimentally. So, here I speculate. Certainly, the shape will depend on the local distribution of temperature, which will vary with the properties of the surface, the thickness of the film, and other factors, but I focus on the influence of the surface.
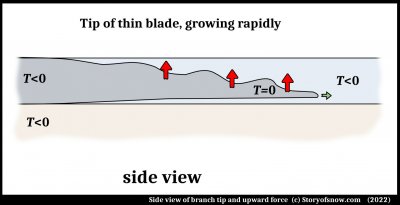
At the ice-surface interface, the ice has strain. It overcomes this strain when it first forms due to the surface becoming sufficiently cold. After the first ice freezes, the latent heat of freezing warms up the region and strong temperature gradients can form. An argument can be made that the resulting blade tip grows out ahead of its attachment to the surface as sketched.
That is, there is a very thin region of water under the tip of the blade. One study (3), in which the melt region was a large volume, noted that the initial growth of ice dendrites was up into the melt and away from the surface. In this case, the ice is confined at the top, so it cannot deviate far from the surface. I had pointed out how thin melt films appear to lie between surfaces and ice in several situations, and produce a force that pushes the ice away from the surface. In this case, I show the force with the red arrows. (The green arrow is the main direction of growth.) What is the source of this force?
My guess is that it is like that in the pebble-caps case. See reference 5 below for that case. A possibly similar case is also that of black ice with bare gaps (6). I do not think it is a simple case of melt expanding upon freezing because this curving behavior has been observed with many other liquids (7), many of which do not expand upon solidification.
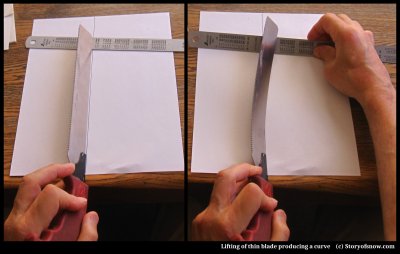
Finally, as to how such a force could twist the crystal orientation as measured in (2), it is as easy to see as grabbing a thin flexible ruler or two:
In the above demonstration, I use a thin handsaw as the thin blade of ice and for an upward force, I pry up the metal ruler. As you can see in the above, if the thin blade has a slight tilt to start, then the upward force does the two things shown in the right image: 1) it bends the branch to the right, and 2) it tilts the orientation. Both of these agree with the measurements in (2), at least qualitatively.
Clearly we could use more systematic experiments of film-frost growth. It has been 51 years since the latest one that I know about (7). In part 2, I will apply these ideas and more to the case of the striped-tail film frost shown at the beginning.
--JN
References and notes:
1) https://www.storyofsnow.com/blog1.php/choppy-waves
This describes how hoar frost forms (Jan. 2012).
2) C. A. Knight: Curved Growth of Ice on Surfaces. J. Applied Physics, 33, 1808-1815 (1962).
Looks at the crystal orientations of the branches, some rules about the sidebranches. Includes growth into water with solutes.
3) P. R. Camp: The Formation of Ice at Water-Solid Interfaces. NY Academy of Science, 125, 317-343 (1965).
Looks at growth rates along several surfaces, several patterns, and growth away from the surface.
4) https://www.storyofsnow.com/blog1.php/hair-ice-on-wood-and-pavement
This describes the role of capillary forces from a thin film of water between a surface and ice, such as on hair ice and pebble caps. The article mentions application to curved growth as well (Jan. 2013).
5) http://www.storyofsnow.com/blog1.php/2010/01/27/ice-on-the-rocks
Here I show pictures of ice columns growing atop pebbles (Jan. 2010).
6) https://www.storyofsnow.com/blog1.php/black-ice
At the end, I mention the idea of melt flow under the ice branches to push them up and dry out the regions between branches (Mar. 2012).
7) C. A. Knight: Breeding of Crystal Nuclei by Classical Nucleation: Theory and Some Observations & Experiments. J. Crystal Growth, 11, 201-217 (1971).
This study proposes a mechanism for the curved growth, and examines growth of other substances that also curve.