Category: "Ice Science"
Rime falling from branches
January 24th, 2013While riding my bike home the other day, I saw what appeared to be a patch of light snow.
It was the only such patch around. Looking closer, I could see that it consisted not of snow, but of chunks of partly melted rime deposits. (Note how the pieces are long and narrow, like little icicles.)
Just above were the branches of a huge Douglas Fir tree. Perhaps there had been an over-active squirrel or two up there. Other trees still had their rime. Hard to see why only this tree would have its rime fallen off.
- JN
Rime, freezing fog, and crystalline spider webs
January 22nd, 2013The Pacific Northwest has been foggy a lot lately, but the fog droplets have been subzero, or supercooled. When such fog droplets hit an object, they almost always freeze. The resulting frozen aggregate is called rime. Freezing fogs make rime.
The resulting rime formations may look like hoar frost, or even snow, from a distance. But close up the special features of rime become apparent.
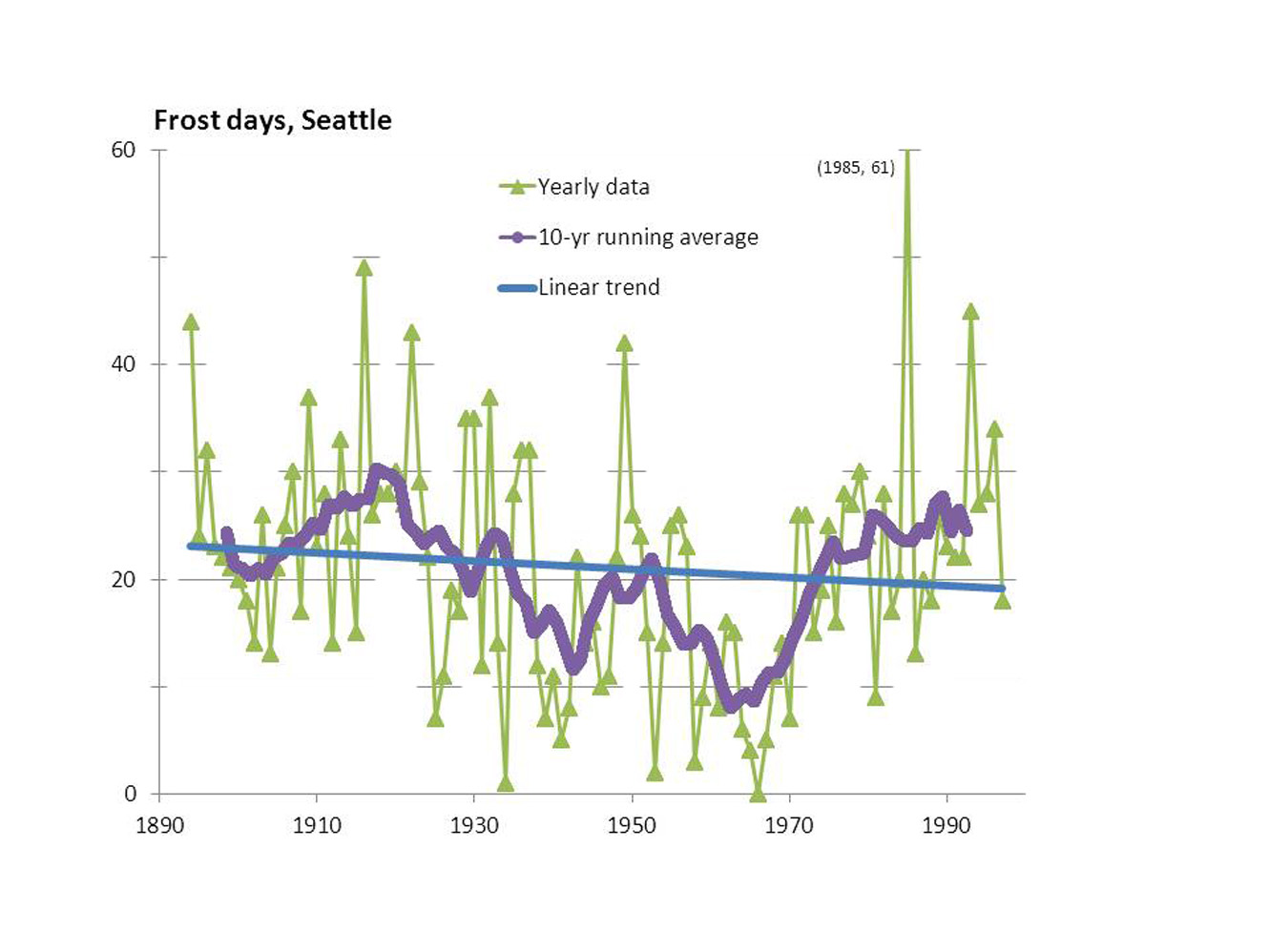
Frost Days and Ice Days: Declining Numbers over the Century
January 17th, 2013David Easterling recently reported in BAMS** that the number of frost days per year is decreasing over the US. A frost day is a day in which the minimum temperature goes below the melting temperature of ice (32 F or 0 C). This doesn't sound good for a frost observer like me. Moreover, the largest decrease, a value of 2.6 fewer days per year per decade, is in my area, the Pacific Northwest. Will frost soon be a threatened species?
His analysis and presentation was based on 1948-1999 data, and moreover, he averaged over multi-state regions. I'm more interested in my local area, King County, Washington, and would like to see both the longer-term trend and variability. I quickly sent him an email, requesting more info. But I was impatient and sought the raw data myself. Following the info in his article, I found the data online***. An added plus: at many stations, the data now goes back another 50 years. With a few simple commands typed into an Excel spreadsheet, I determined the longer-term trend for a station near the University of Washington stadium in Seattle (the only such site in Seattle).
Hair Ice on Wood and Pavement
January 16th, 2013The morning after a rapid cool-down, I found hair ice on an alder log.
From a distance, it looked unnaturally white, like it was a bit of discarded cotton or white paper, but the closer I got to it, the more incredible it seemed.
Crystal-to-crystal “communication” through vapor and heat
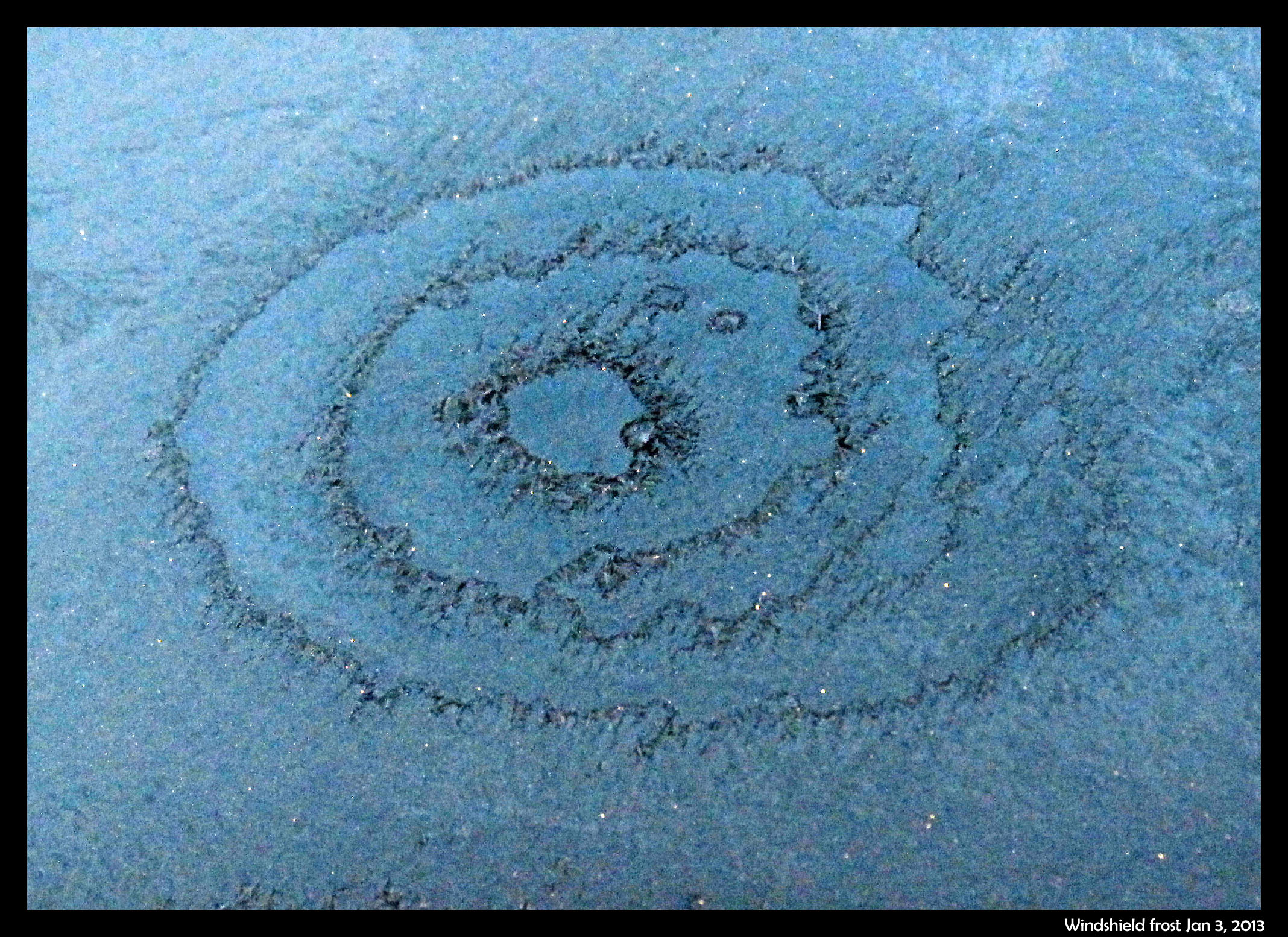
January 5th, 2013Two mornings ago, I saw this on the windshield of a parked car.
The bulls-eye pattern wasn’t centered on any particular feature on the windshield, and there were similar, though less developed, patterns nearby. See them on the photo below.
The dark parts are largely frost-free regions, and thus are regions that dried out during the crystallization event. (It was still dark when I took the shots.) Later, under a brighter sky, I saw a different pattern on the hood of another car, a pattern that I figure has a similar cause. In the hood case, shown below, the dry regions are bright due to reflection of the sky.
Now, about those concentric rings …
I puzzled over a larger such pattern in the Feb. 5, 2010 posting “Ripples”. The causative process that I proposed back then seems consistent with this newer observation, but I will clarify it here.
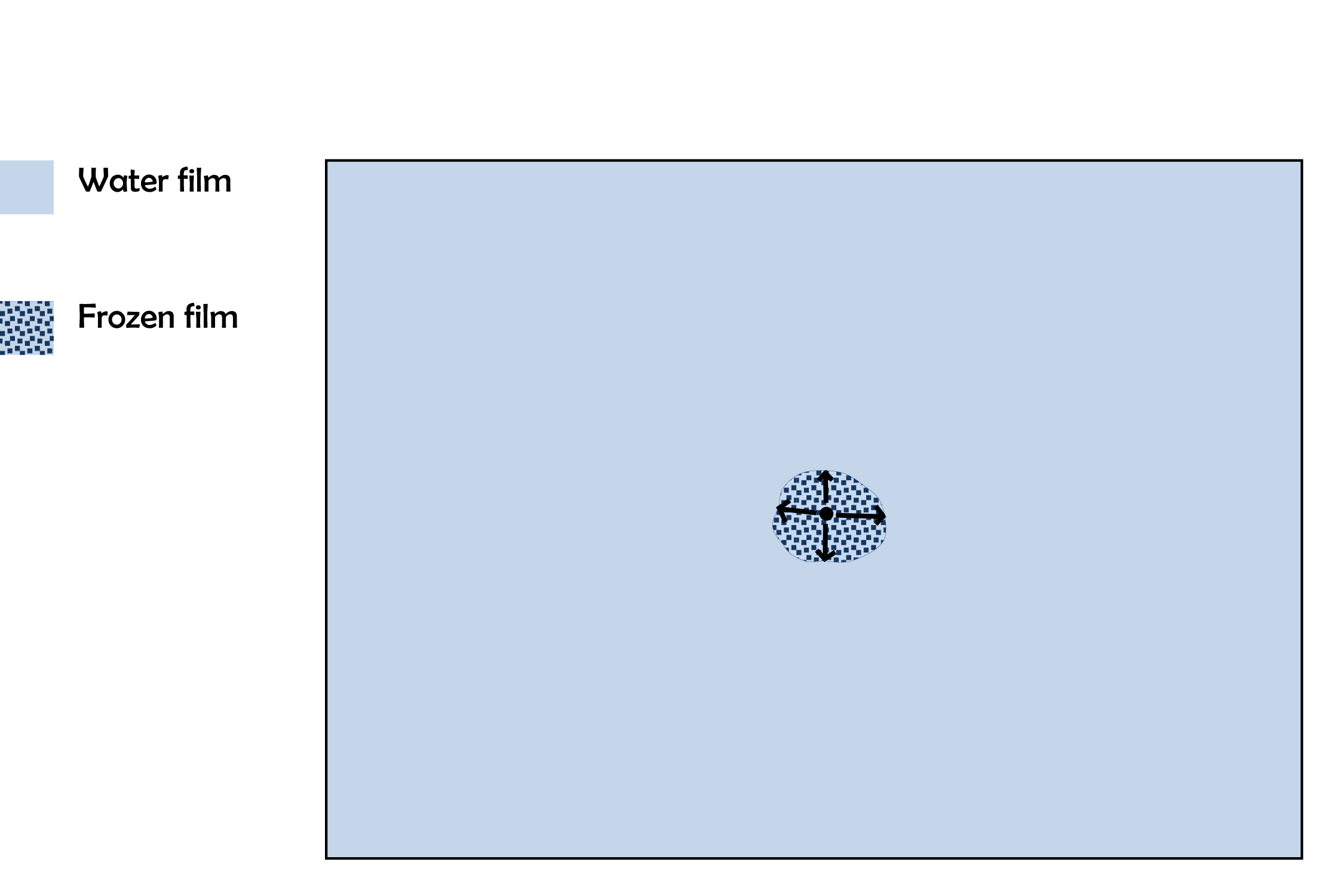
Before the first frost formed, the windshield, though seemingly dry, nevertheless had a thin layer of liquid water. This thin film had cooled to some temperature below freezing. (See the sketch “How hoar frost forms” in the Jan. 11, 2012 posting.) As the windshield and water film continued to cool, freezing was inevitable; the only issue was where. The first such spot to freeze must have had some feature, however minute, that was advantageous to freezing. It may have been a nucleant particle (e.g., a mineral dust grain, a type of bacteria, or even a tiny fleck of ice that wafted in from somewhere else), a slightly cooler spot, or a slightly thicker water film (e.g., from a scratch or indentation). But for whatever reason, the ice formed there first.
Now suppose the ice spreads outward from the nucleation spot in all directions with about the same rate. I suspect this happens when the film is extremely thin, as it would have been under the relatively dry conditions of this day. So, the frozen film grows outward, roughly as a disc. See the sketch below; the black dot in the center is the first place that froze.
Trip of the Ice Man
November 9th, 2012
The "Ice Man" -- that's how a newspaper header referred to me after I gave a recent conference lecture:
A link to the article is here:
http://www.sctimes.com/article/20121023/NEWS01/310230011/Featured-speaker-details-snow-crystals-SCSU-storm-conference
It was a very enjoyable visit to the 7th annual Northern Plains Winter Storms Conference on the campus of St. Cloud State University in St. Cloud, Minn. We had various talks including one about forecasting a storm, the statistics of the snow-to-liquid equivalent ratio (e.g., in some areas about 13" of snow will melt to 1" of water, but regions and storms vary considerably, some being over 70 to 1),and one talk about how a late-season snowstorm might end a locust plague (unlikely, according to the speaker).
My talk described how a simple principle allows us to understand how a wide variety of snow crystal forms originate.
Here's the narrated talk. To view, click the image, then enlarge to full screen:
">
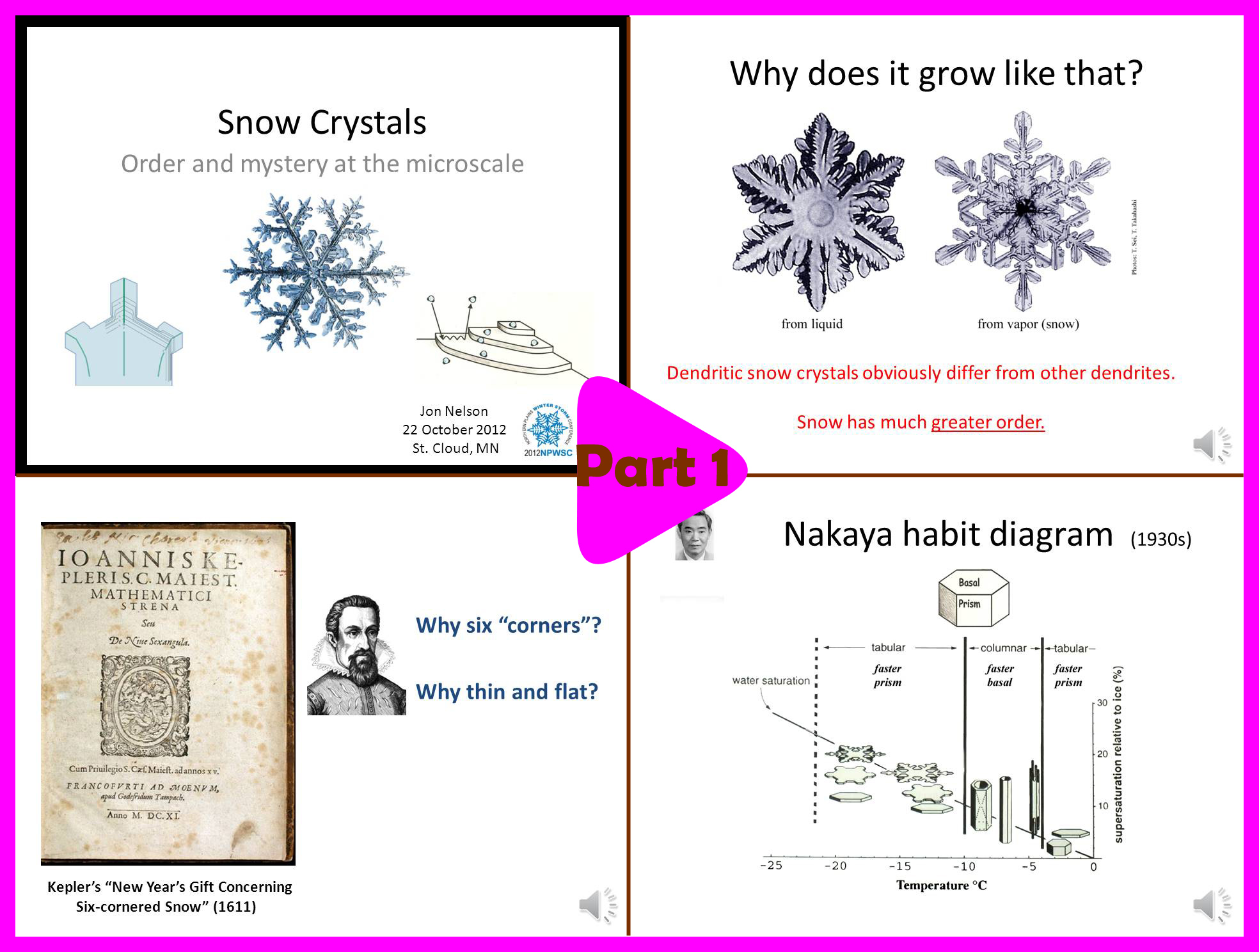
Part 1 (10 min):
-> Why study snow crystal shapes?
-> Some history about snow science.
-> The habit map.
-> Questions that will be answered in the rest of the talk.
">
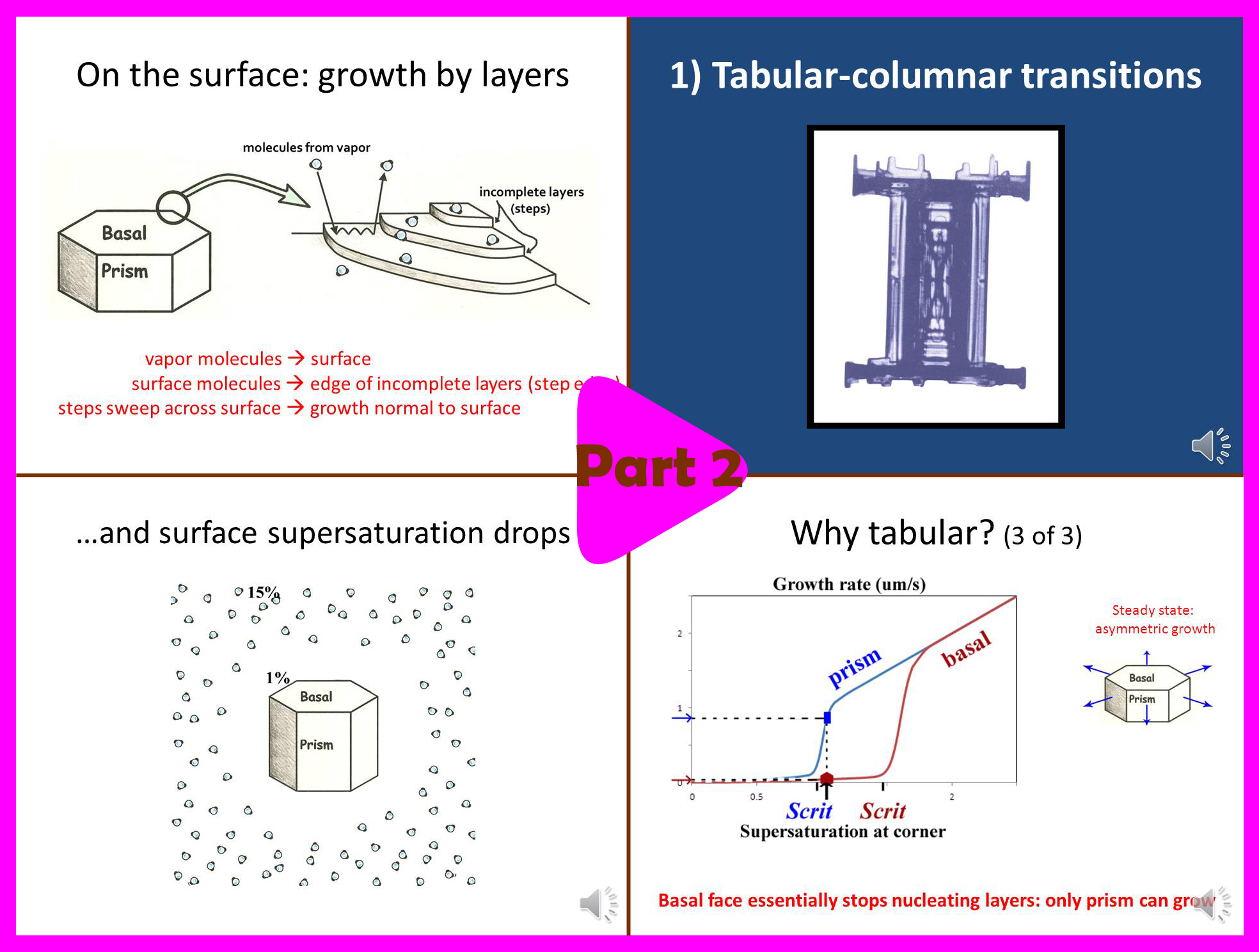
Part 2 (13.5 min):
-> Basics of snow crystal science.
-> Why dendrites grow so thin.
-> Why needles and columns sometimes form.
">
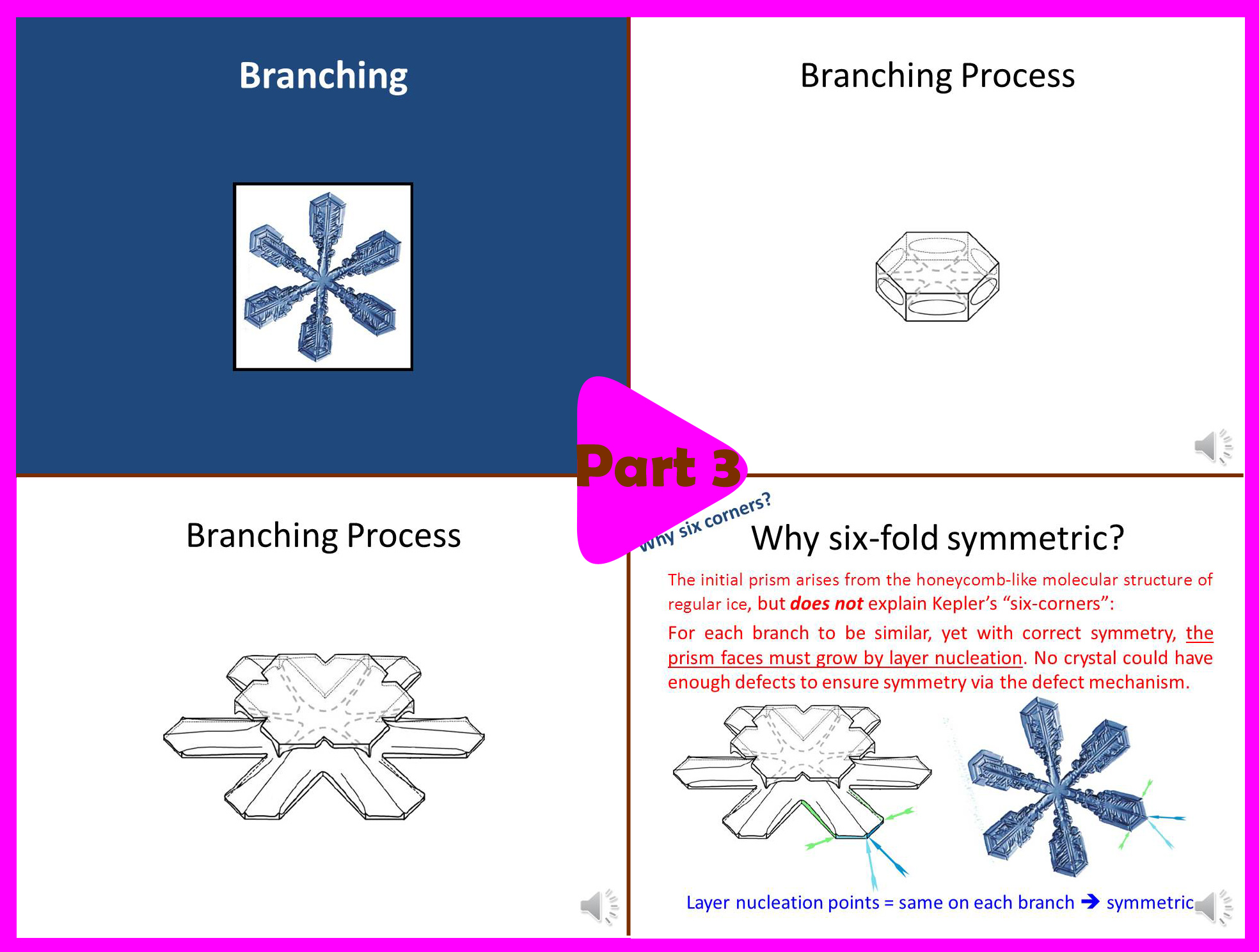
Part 3 (6 min):
-> How the crystals get their branches.
-> Why they are six-fold symmetric.
">
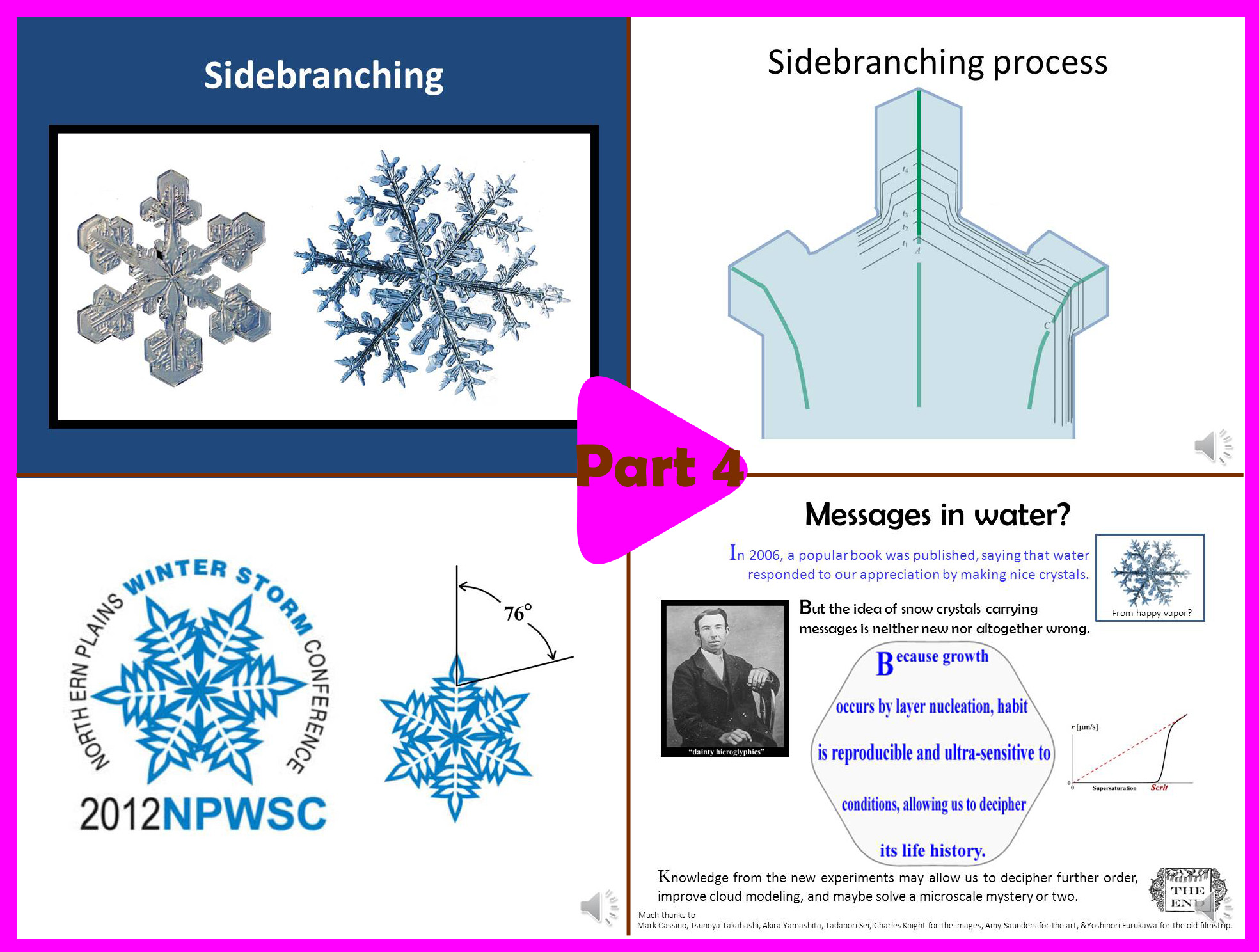
Part 4 (14.5 min):
-> How the crystals get sidebranches.
-> Common errors we make when drawing snow.
-> Why they have so much variety.
-> Mysteries about snow.
-> What are the "messages in water".
It is a scientific talk, so it involves some diagrams and technical terms. But this one is pretty easy. Perhaps the only technical terms are "vapor deposition" and "supersaturation". Vapor deposition happens when water molecules in the air (i.e. water vapor) crystallize onto something, like a snow crystal or hoarfrost. The vapor must be "super" saturated for this to happen. Greater supersaturation means greater vapor density and thus faster growth. The above link goes to the first segment, and from there you can click on the subsequent segments.
On the flight home, I saw a subsun on the clouds below. A subsun is a reflection of the sun from tiny, flat, hovering plate-like (tabular) ice crystals. They are essentially hovering like microscopic flying saucers.
In the photo above, you can see the sun's reflection off the wing on top. The smaller reflection on the clouds below is the subsun. I used to think the subsun was rare, but apparently I simply wasn't looking. This subsun was there in various forms for at least 2/3rds of the flight. I've seen them on most previous flights. More on subsuns in the next post.
- Jon
Here's the abstract to the talk:
snow crystal seminar abstract.pdf
And here are the four parts of the talk in pdf form (from the PowerPoint slides):
snow order and mystery - 1of 4.pdf
snow order and mystery - 2 of 4.pdf
snow order and mystery - 3 of 4.pdf
snow order and mystery - 4 of 4.pdf
Caution!
February 1st, 2012Hoar. It's just a white coating on things, so why does it make everything look more interesting?
I saw this hoar coating on a plastic trash-can lid:
The hoar frost on the lid had various whirls, just like I've seen on the plastic surfaces of car door handles and side-view mirrors. This hoar was a little different though in that the crystals were definitely sticking up and not laying flat on the surface. Nevertheless, the fact that they show a pattern at all, and are not just randomly oriented, means that there must have been a liquid film of water that first froze to the surface. The film froze, producing a pattern of crystal orientations on the surface, and these orientations were not revealed until the hoar frost grew. Hurray for hoar!
Here's another warning:
The hoar crystals are longer on the raised lettering, particularly near edges. This is not because such places are further from the ground, but because they have more radiative cooling (due to their more expansive view of the sky) and can stick out into regions with a greater density of water vapor molecules.
If you click on the images, you can see the crystals a little better. But I forgot my tripod on this particular morning (I took the shots after I got to the office), and so the images aren't as crisp as my other close-up shots.
- Jon
Slush Fingering and Other Pond Patterns
January 19th, 2012Here in the Pacific Northwest, we just had our first snowfalls of the season. On the weekend, we had 1 – 2 inches. This was followed on Wednesday by what the Seattle Times newspaper was calling a “megastorm”. But in the end, most areas in the area got only a few inches. Here in Redmond, we had 4 – 5 inches. And though the temperature barely dipped below freezing, I had several opportunities to observe snow patterns on the neighboring pond.
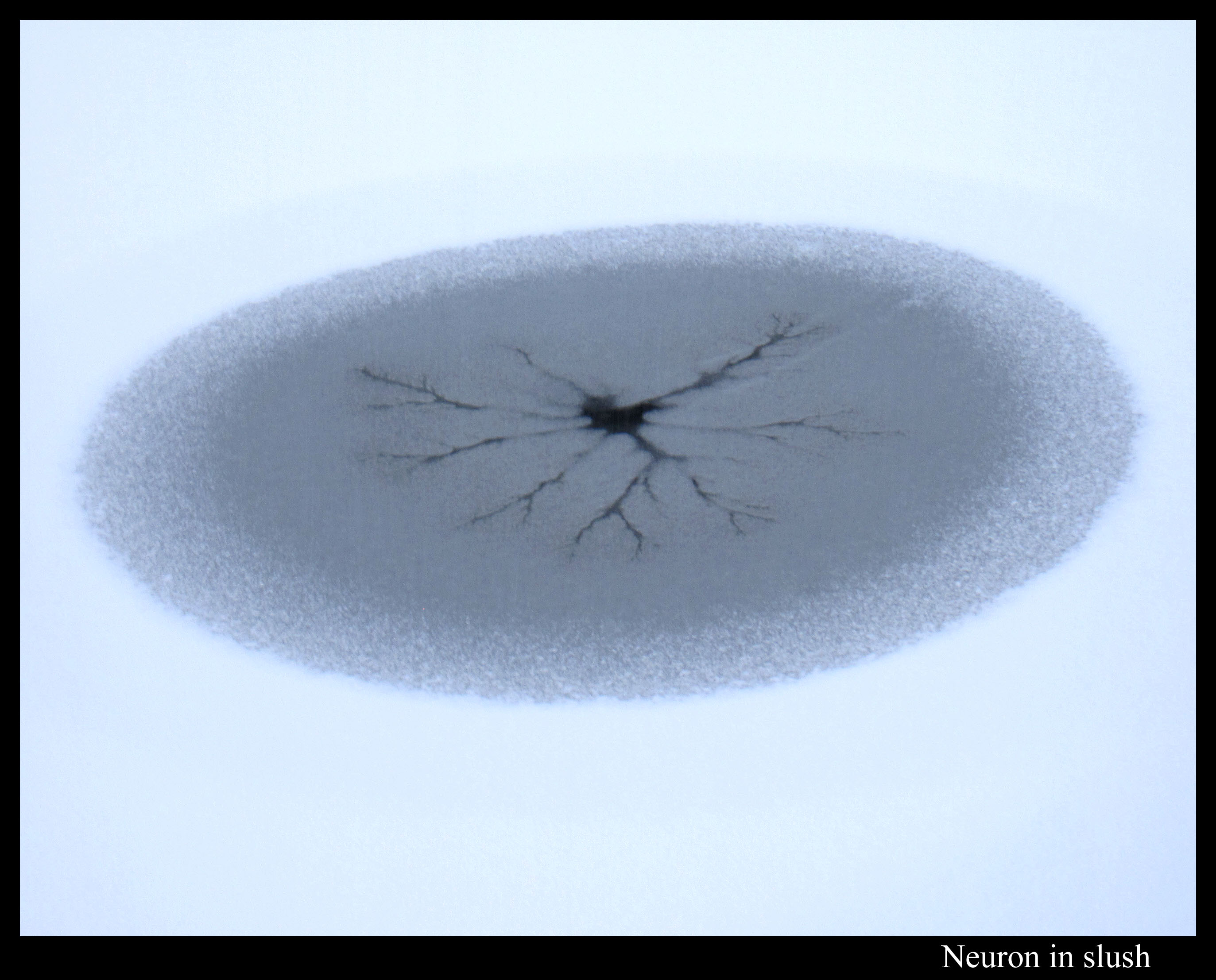
Or maybe I should say slush patterns. (Slush is a roughly uniform mixture of water and snow or ice.) Take the viscous fingering pattern I mentioned in my pond-ice post of a few days ago. Here is a similar type of pattern, except this looks like a neuron dendrite inside an egg.
Unlike the Boulder ice in that previous post, the ice layer on this pond was way too thin for me to walk on. Perhaps that’s a key to understanding why the patterns here instead had darker regions near their center. I don’t know. Anyway, the pond had a few such regions in which the water created dendrite-like fingering.
On the first snowfall, the pond had more circles with dark centers, but perhaps because the amount of snow was less, there was no discernable fingering near the centers, just dark centers caused by more uniform flooding.
In general, the basic layering was liquid below, clear and solid ice above, slush (or frozen slush) on top of the ice, and snow on top of the slush. (There can also be slush under a layer of solid ice.) The first snowstorm, though it had less snow, was preceded by colder weather. So, the clear ice was a little thicker and the slush layer thinner. Between the snowstorms, the ice melted and for awhile we had just slush on top of the liquid water.
The circular and fingering patterns arise when water gets pressed out of a small hole in the ice. The water then floods the ice as sketched below.
Snow on a Freshly Frozen Pond
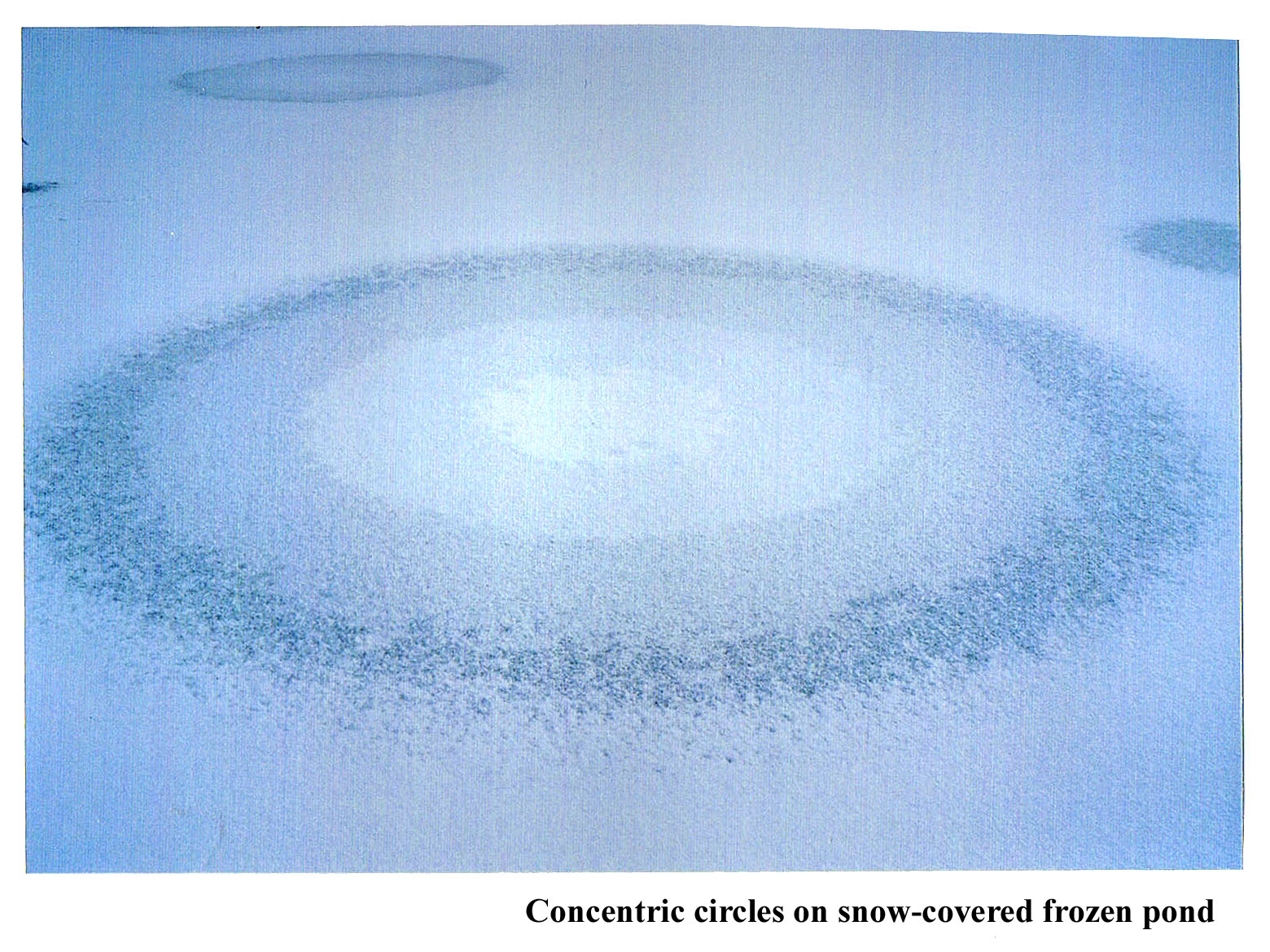
January 15th, 2012Back when I was doing post-doctoral work in Boulder, Colorado, Charlie Knight, the head of my lab, introduced me to strange ice phenomena. The most memorable one happened after the weather had been sub-zero for a few days and then we got some snow. When this happened, we stopped work and drove out to some shallow ponds to look at the patterns on the surface. Sometimes odd, concentric circles formed.
To see the size of the rings, check out the overview.
The guy in the background is Charlie. He is sawing through the ice to get a sample. Behind him are two visitors who came out with us that day. The ice was about 2" thick, if I remember right, and would make some cracking noises sometimes as we walked on it.
I don't know if he figured out the cause of the pattern. I never made any progress in understanding it. Anyway, what seems to happen is that water gets pushed out through a small hole in the ice, and the water apparently spreads out in a circular region. But why does the lightness of the ice change in nearly equally spaced, discrete steps? And why is it whitest in the center?
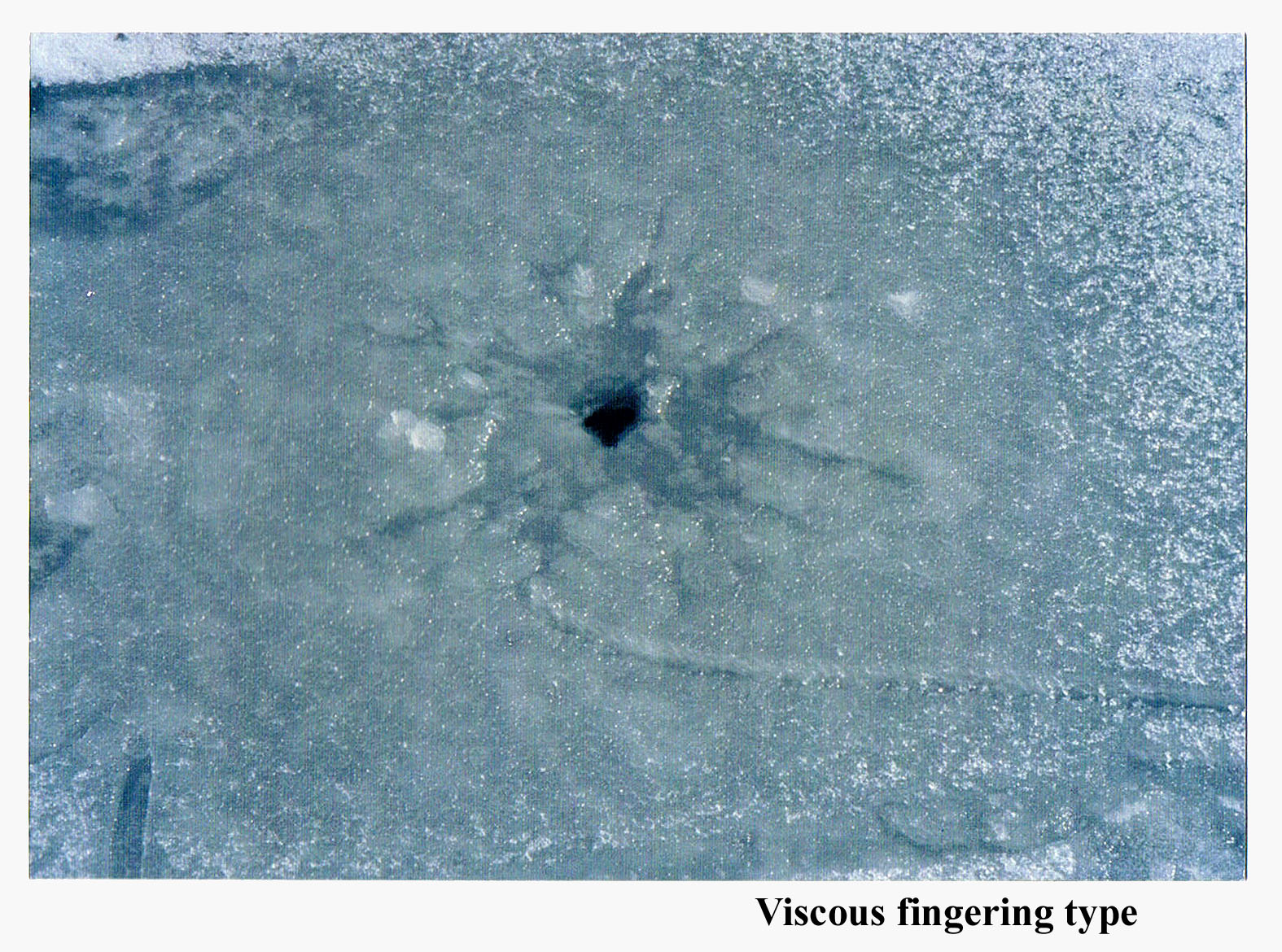
I figured that if water flooded over the ice in discrete steps (day-night temperature fluctuations, as we think happens with the pancake ice?), then the region in the center would be the darkest, not the whitest. For example look at this counterexample.
This shows the hole where water comes out, but the water floods outward in a ragged fashion, not like a concentric circle. This type of flow has been studied a lot in the laboratory and has the technical name "viscous fingering". Anyway, notice that the region near the center is darker, not whiter.
Any ideas about the concentric circles?
--Jon
Fun with windshield ice
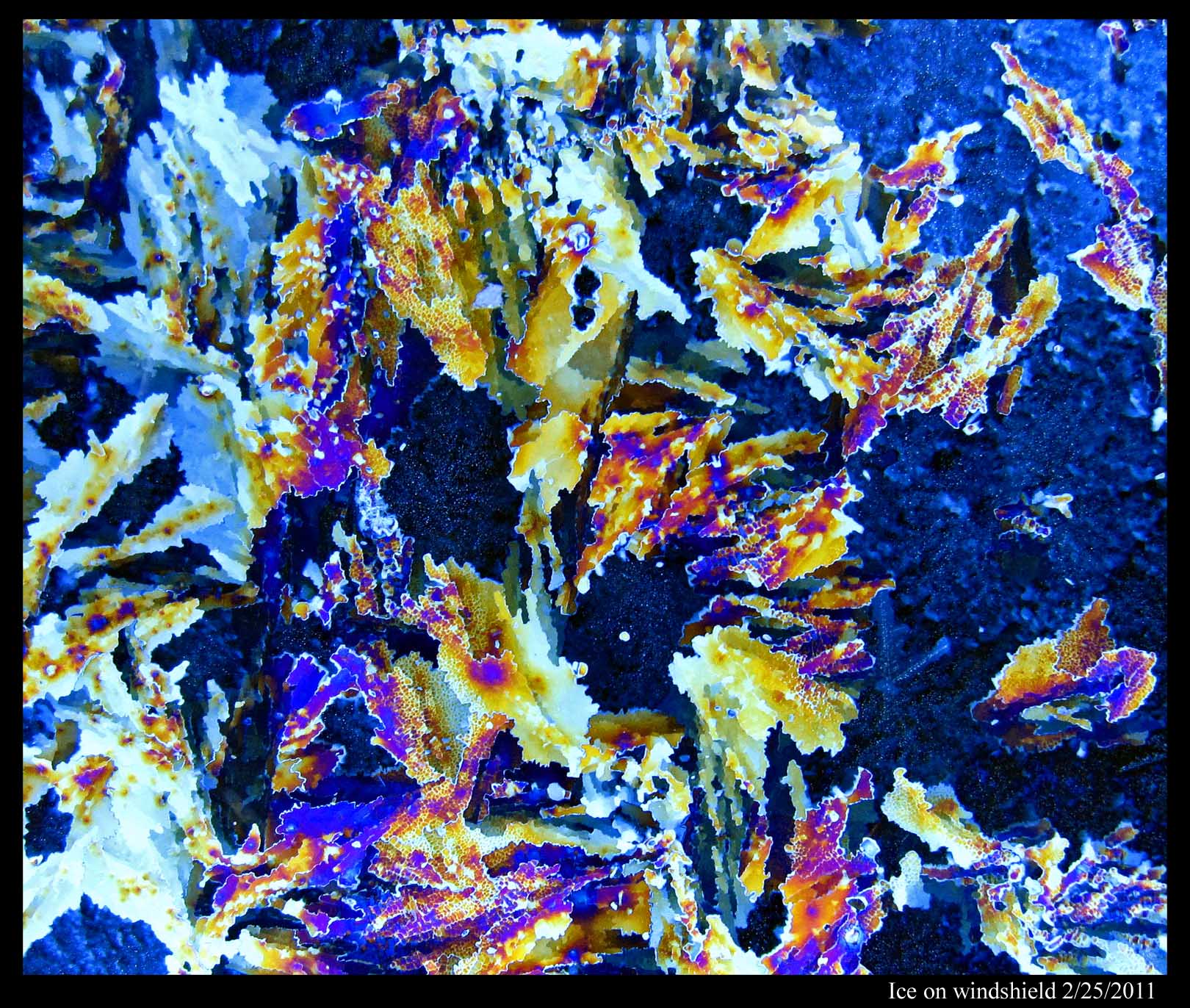
January 11th, 2012In my last post, I pointed out that you can determine the crystal orientation in a film of ice by looking at hoar-frost that sprouts from its surface. Here's another way, but it only works if the ice is on glass. For example, here's ice on my windshield, as seen through crossed polaroids:
To get colors, the ice must be sufficiently thick. You can play around by spraying a mist of water onto your windshield (or some other sufficiently cold pane of glass), letting it freeze, looking through the polaroid sheets, and then spraying some more if you don't see colors you like. One polaroid sheet must be in front of the ice, the other behind the ice. And you need to cross the sheets. You can tell when the sheets are crossed because a bare pane of glass will be black between crossed polaroids.
Two things determine the color: the thickness of the ice and the crystal orientation. So, the boundary between different colors marks the boundary between different crystals. Black regions usually mark regions where the crystal is "basal" orientation; that is, you are viewing the ice crystal lattice from the same angle that you are viewing the nice dendrite crystals that Mark posts here. And where the color changes gradually, you are seeing where the ice thickness is changing gradually.
Of course, don't try this while driving!
-- Jon