| « The Curious World of Ice and Snow: Part 2 of 3 | Some "Inexplicable" Snow-crystal Features: Applications of Lateral Growth » |
The Curious World of Ice and Snow: Part 1 of 3
In 2012, I gave a "science cafe" talk with a local series sponsored by the Pacific Science Center, KCTS public television, and Science on Tap. The title was "The curious world of ice and snow". The location was a bar in Kirkland, but open to all ages. When I showed up with my family, they tried to seat us in the backup room, the regular room having filled up, but I said "Oh, well I'm the speaker" and they kindly created a space for my family in the regular room. I was indeed surprised at the crowd. People are apparently more interested in ice than I thought. (Hmm, but where are they when I post here?)
Click on any image to see an enlargement.
The basic structure of each talk was to give a lecture of about 30 minutes and then allow up to an hour (I think) for the Q&A. In my excitement, I had created 41 slides, in retrospect too many for the allotted time.
Given all the time spent preparing the slides, I hope that by posting them here that even more folks can enjoy the images and discussions. But, instead of unloading all of them on you at once, I will break the discussion into three sections. By adding the following table of contents, each section will have 14 new slides and the total will be 42, which according to Douglas Adams* is a really special number.
The contents of this section is part "1", written in green font.
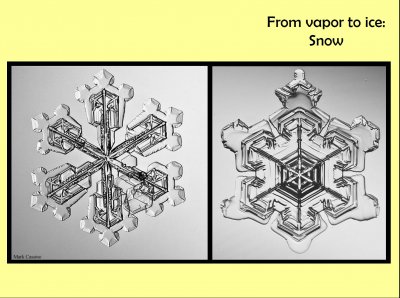
Here are two snow crystals. Snow crystals gain essentially all their mass and shape from the vapor. These two crystals are tabular forms, the left is dendrite with plates, the right is a plate with sectors. Though there is a lot of interior structure in these crystals, much of which hasn't been explored, one thing that few folks realize is how extremely thin these crystals are and how that thinness is unusual in the world of crystal growth.
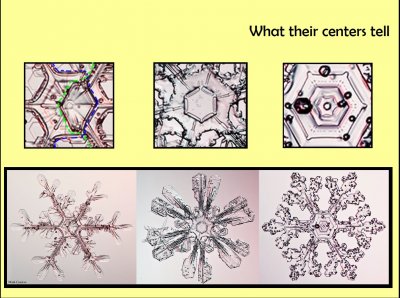
About the structure, consider just one aspect: their centers. These three crystals at the bottom have different centers that indicate a different starting history. The first one started on a frozen droplet, then a thin plane grew off the front side, another plane grew off the back. As they grew, one plane sprouted three branches on one side, say the side marked by the green dashed line, the other plane sprouted three branches on the other side, the blue perimeter here. Overall, it is a six-branched crystal, and you wouldn't think the branches are on different planes. But they are.
The middle one also started on a frozen droplet (as do all snow crystals, as far as we know), but did not sprout the thin planes. Instead, it formed a hexagon-shaped crystal initially, and lather sprouted branches. But this one is unusual for a few other reasons. One appears insignificantly small, where these small circles appear along lines from the center to the corners of the hexagon. We just discovered in the lab how these could form, and their existence suggests that at this initial stage of growth, the crystal briefly started to sublimate away before regrowing. The other unusual thing is the 12 branches, with 6 rotated about 15 degrees from the other 6. This means the crystal structure has type of break in it called a twin boundary. That is, this crystal is type of twin.
This crystal on the right has a small circle in the very center, the initial frozen droplet. In this case, the six branches sprout off the same plane, the other plane just having a hexagon perimeter. These other circles sprinkled around are called rime. The crystal fell through a cloud with a lot of small droplets. When the droplets hit the crystal, they froze instantly, retaining their spherical shape.
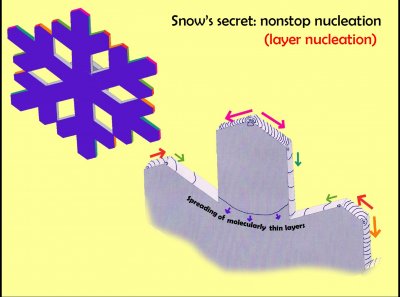
One can understand a lot about the symmetric, recognizable, yet often very intricate and complex forms of snow crystal from this one principle. It is called layer nucleation. In materials science, nucleation is the birth of a new phase, generally a three-dimensional phase like a droplet. But in the case of snow-crystal growth, it is happening all the time in two dimensions on the growing surfaces. Each line here represents the edge of a new molecularly thin layer that is spreading across the surface. Without layer nucleation, you would not recognize a snow crystal. Yet the principle was not understood as the key to growth until the last few decades.
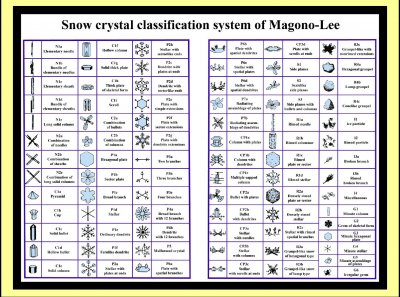
About the different crystal shapes, in the 60s, Magono and Lee expanded upon the previous snow-crystal classification system, making this system of 80 types. (Since this talk, a newer system has been devised with 131 types.) Probably many of these you would not recognize as snow, but in clouds, the less familiar ones, such as this N1d "bundle of elementary sheaths" are more common than these classic snow-crystal forms, like this P1e "ordinary dendrite".
Other than the crystal shapes, snow is known as a material that sticks together. You might not think about that much because it is so familiar, but compare snow to sand. Both are small grains, but try and pack a dry sandball, and it just falls apart. Snow, in contrast, clumps into a snowball. Sometimes, a bit starts rolling down a hill, like in this picture, and instead of sliding, it grows larger. It "snowballs", as they say. These here are called "snow rollers" for obvious reasons. The surfaces readily stick (or sinter) together, and many consider this due to the surface of each snow grain being coated with a thin melt-like layer. The two particles' surface contact each other, and like other wet objects, their melt layers merge and they are held together by the cohesion of the melt (which usually crystallizes at the contact point). Because the ice surface is either more active at warmer temperatures or has more of a melt-like layer, warmer snow clumps better than colder snow. I use the term 'melt' instead of 'liquid' because the melt of ice is pure water, unlike, say, salty water, which is also a liquid.
When it is very cold, like the minus 50 C or so where this picture is taken (near the South Pole), the snow itself will not normally stick together. Yet here are small balls of clumped-up "snow". What we think happens here is that the fast-growing hoarfrost from the surface of the snow develops an electrical structure that causes broken hoar to attract to other pieces of hoar, like a bunch of little bar magnets. The wind-tumbled hoar balls collects more hoar as it rolls, growing larger and ending up like these. The discoverers called them yukimarimo, "yuki" meaning snow in Japanese and "marimo" being a type of plant in Japan that has a ball shape.
The hoarfrost can grow off any solid surface, and you often see the crystals on leaves and grass after a clear night in winter. Hoarfrost grows just like snow crystals, by collecting vapor from the surrounding air. Look close and you will see that nearby crystals have the same orientation. I'll explain that in a moment.
This hoarfrost on a car roof shows this property quite well. Nearby crystals are pointing like hairs in the same direction. But as you go out further from a given crystal, the direction can differ more and more.
Another thing you might notice about hoarfrost is that the crystals are bigger on places that stick up or at edges. There are two reasons for this. 1) Nearer an edge is generally cooler, as it receives less infrared radiation from nearby objects. This might not seem obvious, but if you think about what the view would be like if you were an ant sitting at a given spot, you can consider that the greater the view of the sky, the more cooling you would have. And the more cooling, the more vapor that can be collected. 2) In such locations, the density of vapor is often greater as it is not deleted as much by nearby crystals and the supply is greater.
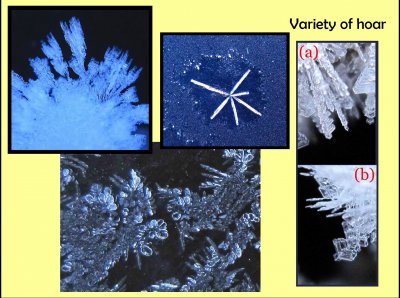
Though snow crystals are known for their variety, hoarfrost crystal have even greater variety. One reason they have more variety is that they can grow even faster than snow. This top middle one is similar to a common type in high cirrus clouds, but here is growing at a much warmer temperature and is much larger. The ones just below are growing with rounded surfaces, which is not known to occur with snow, at least like this.
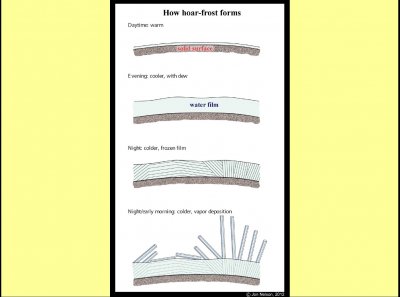
Few people realize that all surfaces outdoors have a thin water film on them, even things that seem quite dry. The film, of course, may be microscopically thin. But when the surface cools, often in the evening, the film grows thicker. If the film temperature goes a few degrees below freezing (32 F or 0 C), ice may form in the film and spread. As the ice grows across the film, the crystal orientation may change slowly, but for nearby points, the crystal orientation is the same. So, when the frozen film starts collecting vapor molecules, causing hoar crystals to sprout, nearby hoar crystals will point in the same direction as shown at the bottom.
This is basically also how snow crystals form, except instead of a large flatish surface, it is usually a microscopic grain of dust. First a little melt freezes, and the rest is collection of vapor.
So, you see there is a very close connection between what we see with hoarfrost and what happens with snow crystals in clouds. There is also a long history of scientists inferring phenomena in clouds from observations of hoarfrost. This picture shows one such case. Originally, this car hood had a liquid film. As it cooled, a few spots froze first. Where they froze, they started growing from the vapor, drawing vapor from surrounding regions. By depleting the surroundings of vapor, the melt film largely dried up near the crystal. Later, in regions that retained a film further from the hoarfrost crystals, the film froze. The temperature being colder, these areas froze quickly, spreading a thin layer of ice everywhere. The end result is the original hoarfrost crystals that grew relatively large by collecting nearby vapor, a dry moat-like region around them, and then many tiny hoar crystals beyond that.
A very similar thing occurs in clouds, except the crystals are falling through the air and the film of water is instead cloudy air with many small droplets.
This ends section one of the Science Cafe talk. Sections two and three will follow. The following cover slide is from the original talk. It shows "ribbon ice", which will be covered in section three.
--JN
* In the Hitchhiker's Guide to the Galaxy, this number was the supercomputer's answer to the meaning of life, the universe, and everything.