| « It’s not a rainbow (it’s better) | Halo in the sky? Uh, I don't see no halo... » |
The new ice-crystal-growth apparatus
After a few years in the making, our new device for growing single ice crystals in a well-controlled laboratory environment is nearly ready. We are just adding a few small accessory pieces to allow us to start testing. I was to describe the apparatus at the cloud-physics conference this month in Boston, and made a poster to present, but decided to opt out. But, having spent a few days making the poster, I present it below.
As with all images here, click on image to see large-scale view.
And below is the same, but in blog format.
New Apparatus for Studies of Nucleation, Deposition, and Sublimation of Cloud Ice
Why design a new apparatus?
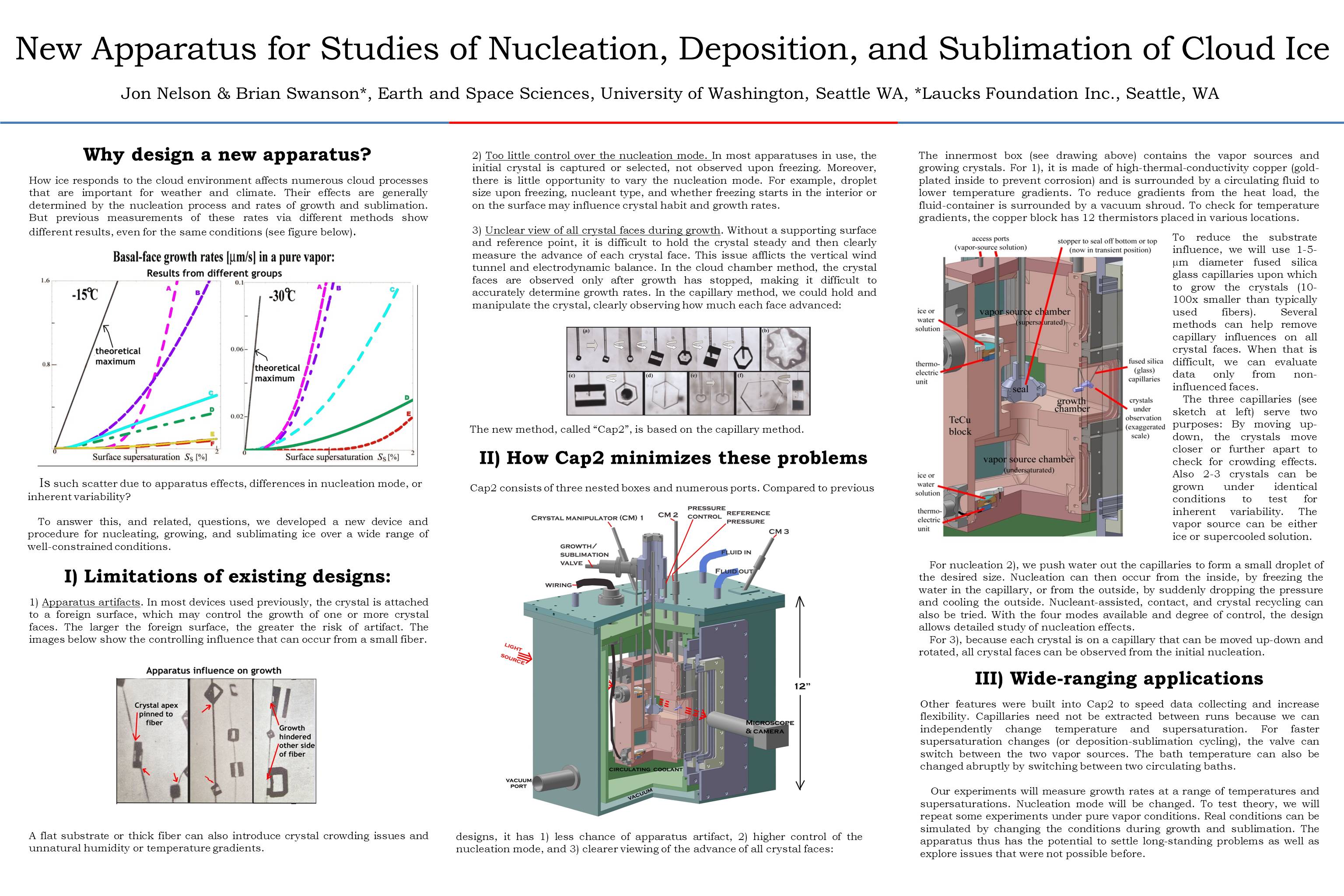
How ice responds to the cloud environment affects numerous cloud processes that are important for weather and climate. Their effects are generally determined by the nucleation process and rates of growth and sublimation. But previous measurements of these rates via different methods show different results, even for the same conditions (see figure below).
Is such scatter due to apparatus effects, differences in nucleation mode, or inherent variability?
To answer this, and related, questions, we developed a new device and procedure for nucleating, growing, and sublimating ice over a wide range of well-constrained conditions.
I) Limitations of existing designs:
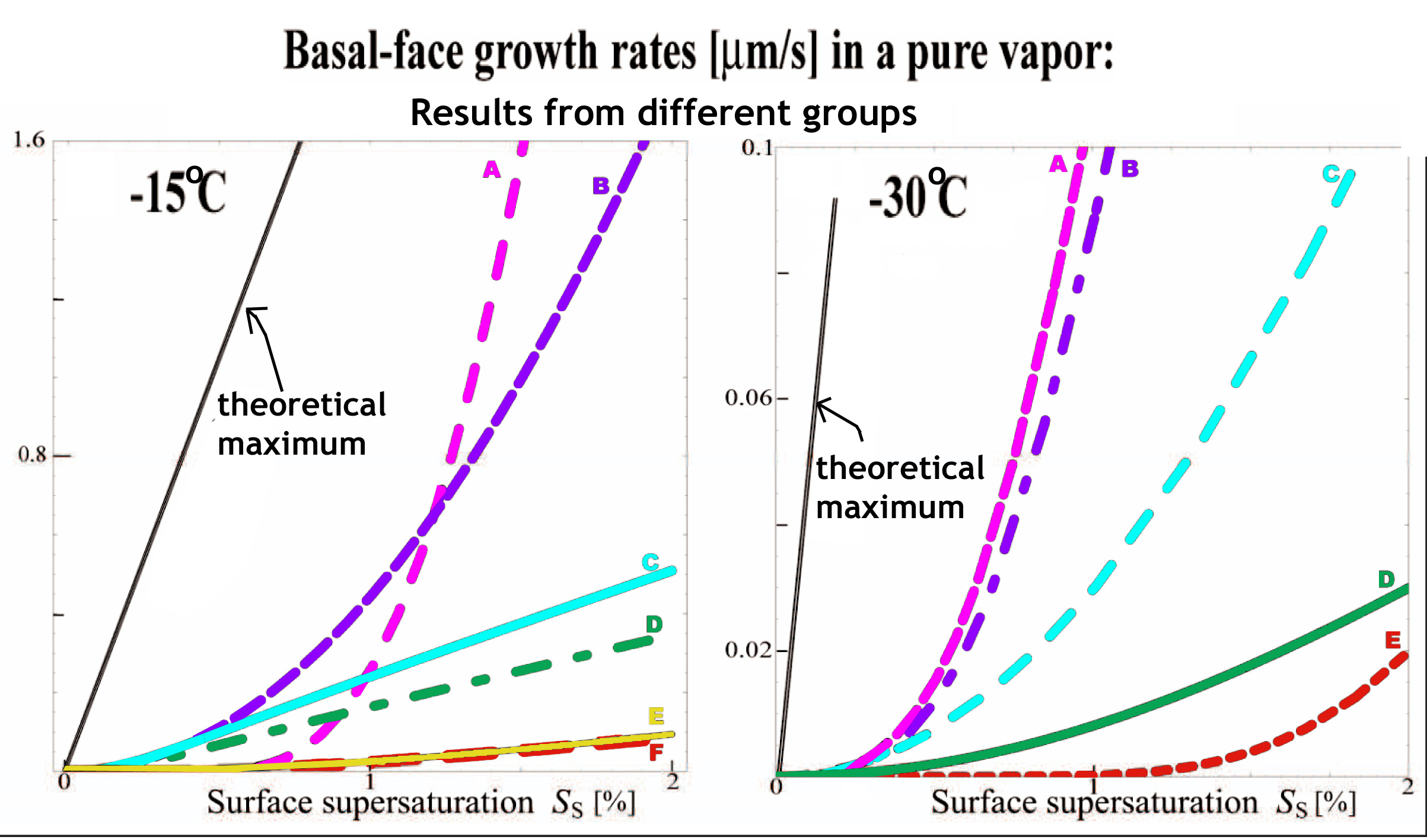
1) Apparatus artifacts. In most devices used previously, the crystal is attached to a foreign surface, which may control the growth of one or more crystal faces. The larger the foreign surface, the greater the risk of artifact. The images below show the controlling influence that can occur from a small fiber.
A flat substrate or thick fiber can also introduce crystal crowding issues and unnatural humidity or temperature gradients.
2) Too little control over the nucleation mode. In most apparatuses in use, the initial crystal is captured or selected, not observed upon freezing. Moreover, there is little opportunity to vary the nucleation mode. For example, droplet size upon freezing, nucleant type, and whether freezing starts in the interior or on the surface may influence crystal habit and growth rates.
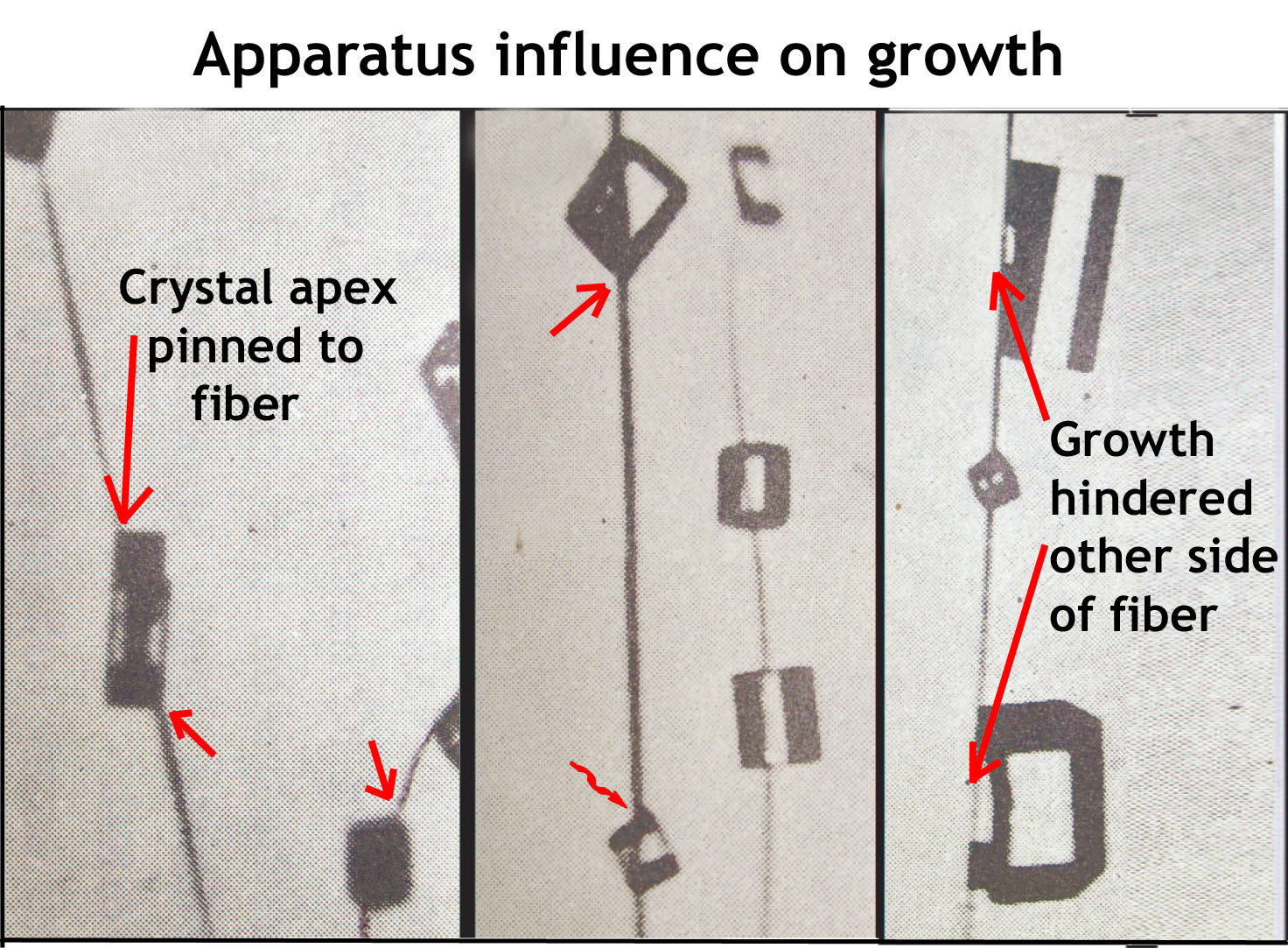
3) Unclear view of all crystal faces during growth. Without a supporting surface and reference point, it is difficult to hold the crystal steady and then clearly measure the advance of each crystal face. This issue afflicts the vertical wind tunnel and electrodynamic balance. In the cloud chamber method, the crystal faces are observed only after growth has stopped, making it difficult to accurately determine growth rates. In the capillary method, we could hold and manipulate the crystal, clearly observing how much each face advanced:
The new method, called “Cap2”, is based on the capillary method.
II) How Cap2 minimizes these problems
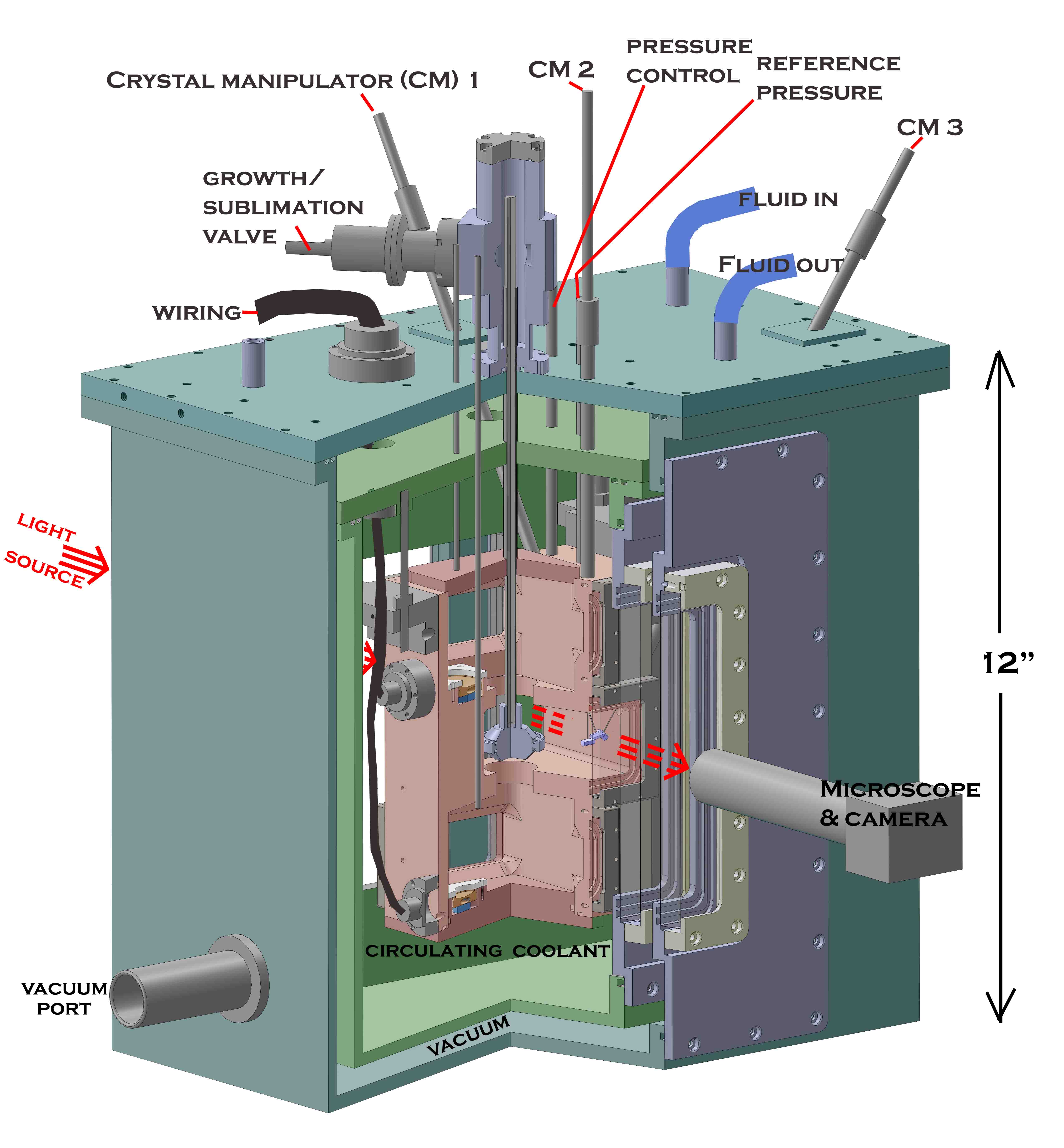
Cap2 consists of three nested boxes and numerous ports. Compared to previous
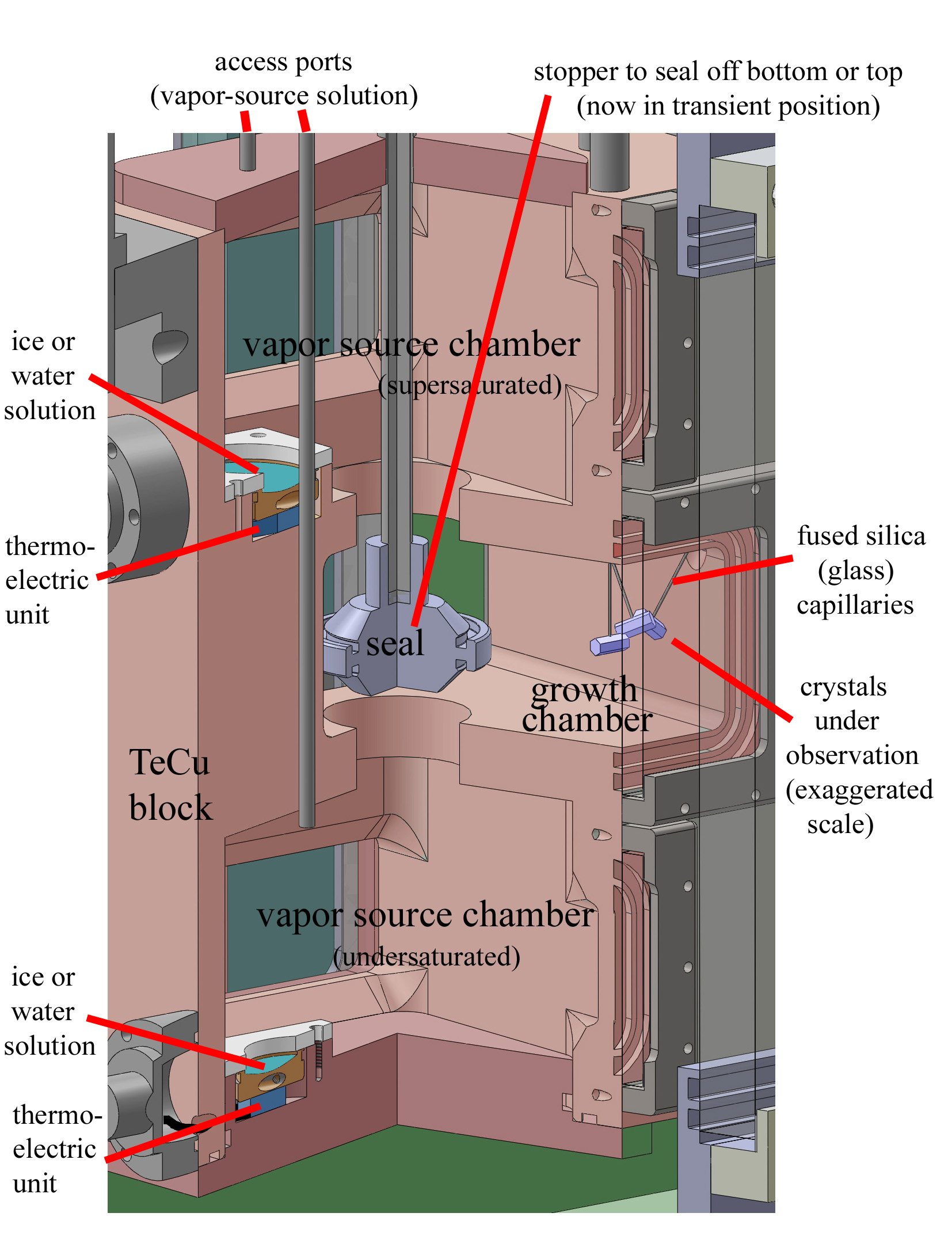
designs, it has 1) less chance of apparatus artifact, 2) higher control of the nucleation mode, and 3) clearer viewing of the advance of all crystal faces: The innermost box (see drawing above) contains the vapor sources and growing crystals. For 1), it is made of high-thermal-conductivity copper (gold-plated inside to prevent corrosion) and is surrounded by a circulating fluid to lower temperature gradients. To reduce gradients from the heat load, the fluid-container is surrounded by a vacuum shroud. To check for temperature gradients, the copper block has 12 thermistors placed in various locations.
To reduce the substrate influence, we will use 1-5-micrometer diameter fused silica glass capillaries upon which to grow the crystals (10-100x smaller than typically used fibers). Several methods can help remove capillary influences on all crystal faces. When that is difficult, we can evaluate data only from non-influenced faces.
The three capillaries (see sketch above) serve two purposes: By moving up-down, the crystals move closer or further apart to check for crowding effects. Also 2-3 crystals can be grown under identical conditions to test for inherent variability. The vapor source can be either ice or supercooled solution.
For nucleation 2), we push water out the capillaries to form a small droplet of the desired size. Nucleation can then occur from the inside, by freezing the water in the capillary, or from the outside, by suddenly dropping the pressure and cooling the outside. Nucleant-assisted, contact, and crystal recycling can also be tried. With the four modes available and degree of control, the design allows detailed study of nucleation effects.
For 3), because each crystal is on a capillary that can be moved up-down and rotated, all crystal faces can be observed from the initial nucleation.
III) Wide-ranging applications
Other features were built into Cap2 to speed data collecting and increase flexibility. Capillaries need not be extracted between runs because we can independently change temperature and supersaturation. For faster supersaturation changes (or deposition-sublimation cycling), the valve can switch between the two vapor sources. The bath temperature can also be changed abruptly by switching between two circulating baths.
Our experiments will measure growth rates at a range of temperatures and supersaturations. Nucleation mode will be changed. To test theory, we will repeat some experiments under pure vapor conditions. Real conditions can be simulated by changing the conditions during growth and sublimation. The apparatus thus has the potential to settle long-standing problems as well as explore issues that were not possible before.
-JN