| « Musings on Bentley’s ‘no two alike’ | Hole in Cloud » |
The Snowflake’s Closest of Kin
[From 2006 through 2012, I contributed annual articles to the annual newsletter "Snow Crystals" for the Wilson Bentley Historical Society. That newsletter is no longer available, so I will repost my articles here, starting with this one from 2006.]
Wilson Bentley is well known to readers here for his photomicrographs of snow crystals. Snow, however, was only one of the many ‘water wonders’ that held his fascination (ref. 1). Some of these wonders were made of liquid water, such as dew, and some, like the snow crystal, were frozen water (ice).
The frozen type he called “The snowflake’s closest of kin”, and they included hoarfrost, rime, windowpane frost, and ice flowers (2). To obtain photographs of any of them with the quality obtained by Bentley is difficult even now, which is yet another reason to admire Bentley’s skill and perseverance.
On the inside, these ‘kin’ all have the same crystal structure. But they appear different on the outside, largely due to the different ways the water in the surroundings gets to the ice surface. There are many distinct kin because the surrounding water can be in various states (i.e., ice, liquid, and vapor) and there are many ways that each state of water can get to the ice surface. I’ll focus here on snow crystals, hoarfrost, rime, windowpane frost, and ice flowers. These forms are commonly seen by many of us, and have been observed by people for a very long time. So it is easy to think, as I probably once did, that they are well understood by science. But this view is quite mistaken. Yes, we know they all consist of H2O molecules and we know something about the structure of ice, but how exactly they form contain many mysteries. I’ll describe briefly what Bentley thought of them, and what I think is known and not known about them.
Snow crystals These were the first ice forms that Bentley studied, and they always fascinated him the most. Snow forms in the atmosphere, either directly on a microscopic dust particle or on a frozen droplet, and grows by the bombardment of water molecules from the water vapor in the air. This growth mode is called growth from the vapor. However, this ideal snow form is often ruined by two other growth modes. When many snow crystals strike each other and stick together, the result is a snowflake (growth by aggregation). When cloud droplets strike a falling snow crystal, the nice crystalline pattern gets covered up, eventually turning the snow into a white blob called graupel (growth by riming). So, if you have tried examining snow crystals yourself, you have probably found, as I have, that most snow looks much different from those in Bentley’s photomicrographs. One often finds snowflakes, partly melted snowflakes, graupel, or tiny pieces of ice that are hard to identify without a microscope. Sometimes though, the conditions are ideal for producing beautiful crystals. If you are lucky enough to experience such a snowfall, then you will understand Bentley’s devotion to snow crystals and why he said (3):
“Every crystal was a masterpiece of design; and no one design was ever repeated.”
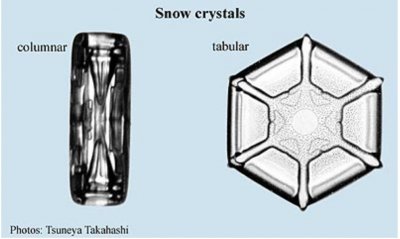
Meteorologists classify these ‘designs’ into 80 types. [[Note, the 80 was the case in 2006, now the classification has 121.]] However, most of these are one of two basic forms; the tabular form, in which growth is fastest on the six “corners” of the ice structure (Bentley’s book, ref. 2, contains mostly this form), and the columnar form, in which growth is fastest on the top and bottom. In addition to these forms, there are also hybrid types that contain both tabular and columnar parts and irregular types such as the seagull type. The biggest unsolved mystery of snow is to determine why the tabular form occurs at some temperatures and the columnar form occurs at other temperatures.
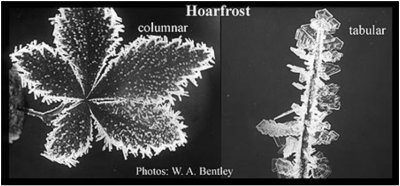
Hoarfrost Hoarfrost refers to the mass of white, hair-like pieces of ice that grow outward from cold objects in cold air. (The word ‘hoar’ is an old term meaning white- or gray-haired.) One can see hoarfrost on blades of grass and leaves after a cold night (pictures below). Hoarfrost is very closely related to snow because it also grows from the vapor. The only substantial difference between hoarfrost and snow is that hoarfrost has grown on some solid object such as grass and leaves. Also, like snow, hoarfrost comes in two basic forms: columnar, which are needle-shaped, and tabular, which have a flat, hexagonal, plate-like shape. The needles form in relatively warm temperatures (yet still sub-freezing), whereas the plates form at lower temperatures. As with the snow crystal, the reason why one temperature results in columnar forms whereas another temperature results in tabular forms is largely unknown.
Hoarfrost crystals are also known to experience some as-yet-unknown type of electrical charging. In this sense, Bentley was right when he wrote (4) “…and there is a play of tiny electric charges about and upon [the snow crystals]…”. Some scientists now think that the electrical charging of growing hoarfrost is closely related to electrical charging of snow crystals, which appears to be important for the charging of thunderstorms. However, the electrical nature of frost and snow remains a research topic with many mysteries.
Rime At a distance, rime looks like hoarfrost, but closer inspection often reveals that its ‘hairs’ are thicker and sometimes packed tightly together into a comb-like structure. Rime forms on an object when the object is exposed to a droplet cloud or fog that is below freezing (32 °F or 0 °C). Thus, the ice grows mainly by the bombardment of small droplets of “supercooled” liquid water, which freeze into ice as soon as they contact the rime. The reader may be surprised to hear that the droplets are below 32 °F, but such supercooled droplets are the norm in clouds above –40 °F, not the exception. Bentley thought that supercooling was due to electrical charges in the water. Although water does indeed have electrical charges, as anybody who has touched an electric fence with a wet stick can attest, but we now know that supercooling is due to the strong surface forces on ultra-microscopic pieces of ice in the liquid that suppress their growth. As clouds of droplets are relatively common on mountains, rime is often seen on ice-cold mountains. When the drops are large and the temperature just barely below freezing the rime becomes smooth and clear. This is called glaze. (See pictures of rime and glaze above.)
Cloud scientists would like to know how the structure (density, bumpiness) of rime depends on the temperature of the air, the windspeed, and the number and size of the droplets. But these things are poorly known. Moreover, the exact ways that the droplets freeze upon impact are largely unknown. Another complication is that vapor growth also occurs on rime. If we could understand the rime structure, we might gain a better understanding of thunderstorms as well. This is because rime also forms on snow crystals as they tumble about in a thunderstorm, causing the crystals to become graupel particles, which, like snow, are crucial for thunderstorm charging. We know that when a snow crystal rebounds off a graupel particle, some electrical charge is passed from one to the other. This charging ultimately produces lightning, but exactly how it works remains a secret of nature.
Ice flowers (Tyndall figures) According to Bentley, there are several forms of ice with the name ‘ice flowers’. In his book he describes two such flower-like forms (ref. 5). One type is now called “Tyndall figures”, named after the Irish physicist-naturalist John Tyndall who first described them. These flowers, pictured below, form when light from the sun, or a bright lamp in a laboratory, is absorbed in the ice, thus melting a little bit of ice within a larger slab of ice. Now ice has the somewhat uncommon property that it floats on its own melt; that is, ice is less dense than liquid water, or, to put it another way, a given weight of liquid water takes up less space than the same weight of ice. Therefore, when the light melts a little ice within the larger piece of ice, a little bit of space is left over that is filled with water vapor. In this way, a Tyndall figure is born. As more ice melts, the vapor-filled region expands outward like a six-petaled flower. Surprisingly, their growth form indicates that some ice inside has been heated slightly above freezing; that is, it is superheated ice! Curiously, this form has seen very little study. In some ways, the Tyndall figure is like a “negative version” of the other type of ice flower: the “ice flower on water”, to use Bentley’s term.
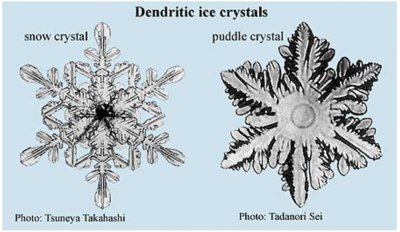
Puddle crystals Ice flowers on water are ice crystals that grow in liquid water. I like to call them puddle crystals because you can sometimes see them in a puddle on a cold day. A puddle crystal looks somewhat like a dendritic (‘tree-like’) snow crystal, but if you look closer in the images below, you will notice some differences. For example, the puddle crystal does not have many interior lines like the snow crystal. Also, the perimeter of the puddle crystal is curved, not straight like many parts of the snow-crystal perimeter. This type of curved ‘tree-like’ growth also occurs in other materials, and, partly for this reason, it has been (and continues to be) studied far more heavily than snow crystals, frost, rime, and Tyndall figures combined. As the snow crystal is more complex than the puddle crystal, and forms in far greater variety, we can be sure that snow will keep scientists stumped for many years to come.
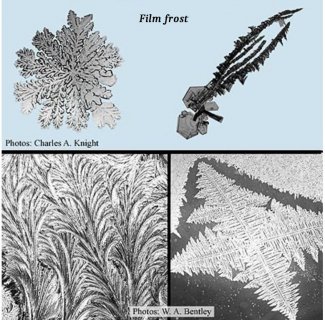
Film frost Unlike hoarfrost and rime, film frost does not stick out from the surface like stiff hairs, but instead grows mostly across the surface like drawings on a sheet of paper. The name reflects how this ice arises from freezing of a thin film of water. The number of pictures in Bentley’s book (2) that are devoted to film frost is second only to that of snow crystals. It is easy to see why; film frost produces a huge variety of patterns, with some looking like a collection of small flowers, some like jagged spikes, some like artistic swirls, some like straight ferns, some like curved ferns, and some like snow crystals. Some of these patterns are shown below.
Bentley carefully studied and classified the types of film frost, perhaps more than any other scientist then or now. And yet, as far as I know, the reasons why film frost has such variety are still not known; indeed, the way that the ice forms and grows on windowpanes is poorly known. However, we do know that glass surfaces generally have a very thin layer of liquid water on them. The curving type of pattern is thought to be due to the freezing of this liquid, which is somewhat, but not exactly, like the freezing of a puddle. You can understand this by noticing that all the frost images below differ from the puddle crystal image above.
In some cases, growth of film frost is partly from the vapor, just like the growth of hoarfrost and snow crystals, except the ice remains in a thin layer against the window. This brings up an interesting story (6) about Wilson Bentley. What happened was this. Bentley observed a clear region of glass between a frost crystal and the surrounding deposit of tiny dew-drops. (You can easily see this for yourself by using your breath to “fog” the glass around a solitary frost crystal on the outside of a car window after a cold night. Also, see the frost image below, bottom right.) He correctly realized that this was a “most singular, and doubtless most important, phenomenon” and supposed that a similar phenomenon occurred in clouds. But, alas, Bentley did not realize the cause.
The cause of this clear region is the rapid evaporation of the dew droplets near the frost, which, in turn, happens because the air around the frost has dried out due to the growth of the frost. As Bentley supposed, the same process occurs in clouds. Indeed it is very important for it results in the rapid growth of snow crystals, which ultimately melt into the large raindrops we receive in the warmer months. Although he did not completely explain the mechanism for the growth of large raindrops, his observational studies of raindrops were important and far ahead of their time.
Closing thoughts Bentley focused his attention on snow crystals because of their beauty and variety. The snowflake kin also have beauty and variety, particularly the film frost (7). Much has been learned about snow crystals and their kin since Bentley’s time, and yet we can still look at them and realize that we are in fact peering into a world that remains largely unknown (8).
--JN
References and notes
1. “Photographing Water Wonders” by Wilson A. Bentley, The American Annual of Photography, vol. 24, pp 84-86, 1910.
2. “Snow Crystals” by W. A. Bentley and W. J. Humphreys, Dover Publications, NY, NY, 1962.
Note that Bentley was aware that 'snowflake' referred to a snow-crystal aggregate (i.e., clump of many crystals) by meteorologists, yet sometimes used this popular term instead of the correct 'snow crystal'. I prefer using the correct term, but follow Bentley's exceptions here.
3. “The Snowflake Man, A Biography of Wilson A. Bentley” by Duncan C. Blanchard, The McDonald & Woodward Publishing Company, Blacksburg, Virginia, 1998. Page 22.
4. Ibid, page 153.
5. See introduction to the book in (2).
6. See “A Discovery Not Made” on pages 133-141 of the book in (3).
7. There are many other ice forms. The relatively common types include the various patterns in the freezing of lakes and puddles, icicles, ice stalagmites, and ground needles (pipkrake), which all grow from the liquid. A less common type, yet quite astonishing in form, is the ‘sap crystal’ or hair ice, which forms from the freezing of plant sap.
8. Thanks to Tsuneya Takahashi, Peter Wolf, Judy Mosby, Michael Sherback, Tadanori Sei, and Charles Knight for the photo images, and to Duncan Blanchard for suggesting changes to an earlier version of this article.