| « Frozen Drops on Web | Why six? » |
Bentley’s Most Singular Observation
[This is the seventh and last in the series of re-posted articles, from 2012.]
You don’t have to look at frosted surfaces for very long before coming across something like the following.
The picture shows a large ice crystal amid a roughly uniform sea of tiny frozen droplets. Between the large crystal and the frozen droplets lies a clear ice-free zone, a dry moat around an island of ice. Sometime prior to 1907, the Vermont farmer Wilson A. Bentley took notice of this moat. Writing in the Monthly Weather Review in 1907, he wrote
"One of the most singular, and doubtless most important, phenomena that occur in connection with the formation of window frost is this: The true crystalline varieties of window frost ordinarily, apparently, repel the minute liquid particles or droplets of water that frequently collect like tiny dew-drops on the glass, and freeze in granular form thereon."
The reason he found the phenomena so important was explained next:
"This phenomenon is endowed with the greatest interest, because the repulsion operates also within the clouds while snow crystals are in process of formation, and hence has an important bearing on forms and structure of snow crystals, and in keeping them free from granular deposits of a like nature."
The appearance of a physical repulsion between the ice and the surrounding droplets (before they froze) is indeed strong. So, in the next paragraph, he speculated about a possible mechanism.
"We can only conjecture as to the cause of this most interesting phenomenon. It may be of an electrical nature. Possibly both the snow and frost, and the liquid cloud or dew droplets, possess an excess of the same kind of electricity, positive or negative. If so they would naturally repel each other."
It was just a brief bit of speculation before immediately returning to his main objective, which was describing his observations of frost. Now the mechanism he described is wrong, but he was right on the mark with the idea that the phenomenon was important. Indeed, the same phenomenon occurs in clouds, playing a major role in the formation of rain.
The real reason for the dry moat lies in the nature of liquid and solid water. At a given temperature, water molecules evaporate from liquid at a faster rate than that from ice. In other words, ice holds onto its molecules a little more strongly than the liquid.
You can picture the scene at the surface as follows: Initially, the glass was covered by tiny dew droplets, with each one of them gaining and losing molecules in roughly equal rates. So, the droplets show no net growth or evaporation. Then one of the droplets freezes. Although the surrounding vapor condenses on the crystal at about the same rate as that on the droplets, the crystal gives up fewer molecules to evaporation. So the crystal experiences net growth from the vapor, while drawing a net amount of vapor from the surroundings. This drawing of vapor to the crystal reduces the amount of surrounding vapor (i.e., humidity) and so the nearby droplets no longer have a balance between condensation and evaporation: they have net evaporation and vanish. In this way, a dry moat forms around the crystal. Later, the other droplets freeze.
The above explanation was probably first proposed by Alfred Wegener in 1911 after he too observed frost. Wegener, though most famous for his theory of continental drift, was by profession a meteorologist. Like Bentley before him, he proposed that the process would also occur in clouds. (Curiously, the next scientific article on window frost in 1939 by Ukichiro Nakaya, Masando Hanazima, and Kenzo Dezuno, also noted the dry moats around large ice crystals, but did not speculate on its origins.) And just as the process occurs in clouds, so too it will occur on other surfaces. For example, the image below shows dry moats on a car hood.
The lesson here, I think, is that study of something common and seemingly inconsequential (e.g., frost) may shed light on a far more important problem (e.g., rain). This is one of the things that distinguishes science from engineering. In this case of the dry moat, Wegener suggested that an ice crystal within a cloud of droplets would grow at the expense of the droplets, and this process would lead to large crystals that later precipitate. In the 1930s, Tor Bergeron expanded upon Wegener’s mechanism, showing how the process would lead to rain. This rain-production mechanism is thought to produce most of the rain in midlatitudes. (For more about the Bergeron rain mechanism and its relation to Bentley’s observations, see Duncan Blanchard’s book in reference 1.)
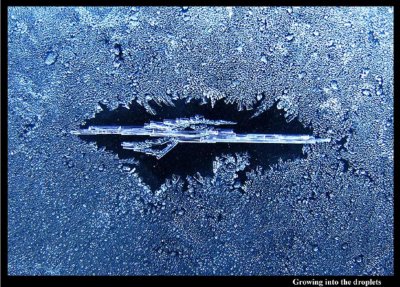
Further observations of the dry moats has other applications to snow crystal growth in clouds. For example, the shape of the moat tells us how the water vapor gets distributed around a growing crystal. The distribution of vapor, in turn, influences the crystal shape and growth rate. Notice in the image below how the ends of the column-shaped crystal lie closer to the droplets. Indeed, the ends have grown into the droplet region. This means that the faster-growing points of a crystal can grow into regions of higher humidity. By growing into regions of higher humidity, they can grow even faster. This effect largely explains why the familiar snow-crystal star is the fastest-growing natural snow crystal. In second place lies the long, thin columns and needles.
So, as Bentley surmised, the dry-moat phenomenon does have an important bearing on forms and structure of snow crystals. His observation was indeed most singular.
--JN
References and notes
1. Duncan Blanchard, The Snowflake Man: A Biography of Wilson A. Bentley, McDonald & Woodward Publishing, Blacksburg, Virginia, 1998. See chapter 11.