| « Thinking Laterally in Crystal Growth…and in Science Publishing | Poster on corner pockets in snow crystals » |
Dark lines in melting snowpiles
Snow cleared from a city street slowly vanishes, leaving lines of "dirt".
(Click on images to view more closely.) Where the snow has a distinct edge, you can see the dark lines are ridges, some of which can be quite sharp:
Why is this?
Cryoconite ridging
This all happens because the snow is vanishing but the dirt is not. The vanishing is actually called "ablation", meaning some combination of melting, sublimating, and evaporating. It is mostly melting in the above case, but it is possible that evaporation helps to form the lines. About the dirt, it is not clear exactly what it is. The term "cryoconite" is used when similar dark dirt falls and clusters on glaciers and ice sheets, so to be specific, we might call it "cryoconite ridging".
At a distance, this cryoconite looks like sand grains kicked up from the road, but look close and the grainy sand appearance gives way to small clusters of dark gunge. Some clusters are in little snow pockets, some appear on top of snow grains. If you touch a particularly thick patch, you will probably find it to be pasty and slimy, with just the occasional mineral grain. In the plowed snow, I think much of the material came from oils and tars on the road, perhaps also tiny bits of rubber, that stuck to the snow before it was plowed. Probably much of the rest is biogenic.
So, what produces these lines? How does the dirt cluster at edges? And why do the edges tend to stay sharp as they ablate?
Ball's normal trajectory model
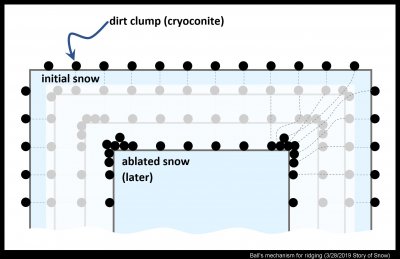
To explain the lines, F. K. Ball in 1954 proposed the "normal trajectory" model. He was interested in a related phenomenon that he and W. E. Richardson (in another 1954 article) called dirt polygons (two years later, Richardson preferred "dirt fringes"). These polygons are similar to the "suncups" one may see on high-mountain snowfields. But instead of looking at polygons, it is more easily understood by considering a rectangle of snow that shrinks via ablation.
In the above diagram, the clumps of dirt, or cryoconite, are initially evenly dispersed on the surface. But when the snow surface ablates, the cryoconite stays in the same spot, essentially moving perpendicular (i.e., normal) to the receding surface. This is why it is called the normal trajectory model. Where the corner encroaches on the clump, the clump stays at the surface, essentially becoming trapped at the corner. Thus, after a lot of snow has ablated, the cryoconite is more concentrated at the corner. See the clumping of circles at the corners, with their trajectories as dashed lines at right. My sketch shows a 90-degree corner of a rectangle, but 90 degrees are not needed. In fact, one doesn't even need a sharp angle, but just a slight convex section.
Here's a sequence of a thin fin ablating while capturing a fir needle and two cone-like fragments at the edge.
So, the observations seem consistent with Ball's mechanism, though it hasn't been tested quantitatively in a careful experiment.
What does cryoconite ridging have to do with coffee?
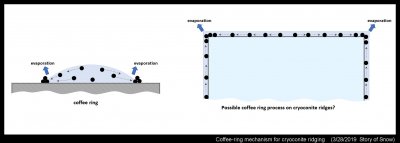
One thing I wonder about is whether an additional process might contribute to the phenomenon. This process we see everyday in spilled liquids, though perhaps we associate it most with coffee spills. It is called "coffee rings" and surprisingly was not explained until 1997. I recall meeting one of the study's authors when we were both new faculty at the University of Arizona. He was surprised at how easy the experiments were and tickled at the attention the study brought. Anyway, the process is simple to describe: The water in the drop flows to the edge of the ring because a) this part is pinned down to the underlying surface (e.g., a table) and b) evaporation is faster at this edge part. So, the coffee particles flow with the water to the edge, but remain trapped there because they do not evaporate with the water. See the diagram at left:
My idea here is expressed on the right side. The surface of the snow has some meltwater. It is just a thin layer, but if the snow is melting it must be there. Probably most of the melt flows into the snow, and percolates down. But what happens when some evaporates? Could the corners evaporate faster like the edges of the coffee ring? If so, then some melt may flow in from the side to replace the lost water. The cryoconite material itself might tend to draw in more water. Inside this thin melt layer will be more small dark particles, like the coffee particles, and they would also flow towards the ridge, further building up the cryoconite. So far, there is absolutely no evidence for this "cryoconite ring" process, but it may be worth testing someday.
Why exactly do the edges stay sharp?
If the dark regions absorbed more solar radiation, they should warm up and melt faster than nearby clean snow surfaces. In this case, a cryoconite ridge would decay and the snow surface would flatten. So, if solar heating was the only process acting, no ridges would form. In fact, there is another heating process at play, and that is heat conducted from the air. The dark regions can absorb solar radiation by the first factor, but also provide insulation against warm air by the second factor. So there are two factors that may compete with each other.
A researcher studying ash-laden snow after the eruption of Mt. St. Helens (1980) found that the first factor, the melt-increase effect from sunshine, occurs when the layer of ash is very thin. As more ash builds up, this effect is stronger until reaching a critical thickness of about 3 mm. As the ash layer exceeds this critical thickness, the effect weakened as the second factor, the insulation effect, starts to dominate. By a thickness of about 25 mm, the melt rate actually becomes less than that for clean snow. Now the ash layer acts mostly as insulation against the heating. A more recent study argues that the critical thickness can be much less than 3 mm when the melting is driven mostly by heating from the air, that is, when it is overcast or the snow is in a shady area.
Does this explain the sharp edges in cryoconite ridging? Here, the cryoconite at the edges seemed rather thin, probably less than 1 mm, and the skies were overcast. So, it is possible that indeed the cryoconite provided heat insulation at the edges, thus reducing the melt rate there while snow away from the edge melted faster. This process would sharpen the edge.
But in another post, I'll show evidence that the melt rate of similarly dirty snow (i.e., same area, same time) was not significantly different from that of clean snow. So, it appears that the cause of the sharp edges has actually not been explained. What then reduces the melt rate at the edge? We might consider the "coffee-ring" or "cryoconite-ring" effect mentioned above. That is, any increased heating at the cryoconite ridge is offset by an increase in evaporative cooling from the above thin-film flow in the "coffee-ring process". Again, careful experiments are needed.
Here is a case of ridges remaining as a snow clump shrinks to about one-fourth its initial size.
In this case, the ridges neither got sharper nor duller. In general, the change in sharpness of a ridge may depend on several factors including the amount of cryoconite.
Finally, one last view of the phenomenon.
What do you think of dirty snow now?
--JN