| « How some snow-crystal centers retain their droplet origin | Akira's Corner Pockets » |
How the water molecules make corner pockets
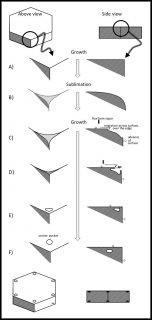
As I mentioned in my previous post, the formation of corner pockets on ice crystals is inexplicable by the standard theory of snow-crystal growth. Akira Yamashita had recently proposed a mechanism for corner pockets, though he had applied it to a different situation. In his case, he observed the early growth stage of a relatively large frozen droplet as it transitions into a faceted crystal. So, his situation dealt with a round crystal becoming sharp-edged. In our case, we started with a large sharp-edged crystal, then made it become round by sublimating (analogous to evaporation, but for a crystal), and finally made the crystal re-sharpen by growing it. The process is sketched below. You may want to open the image in another window and enlarge it.
At the top, you see the simple hexagonal prism crystal in its initial stage. At stage B, the crystal sublimates (we reduced the amount of vapor), meaning that it is starting to shrink. The shrinking starts from the corners, making them become rounded. Then, in stage C, we start growing the crystal again, and the interesting things start to happen.
As the water molecules from the vapor stick to the surface, they remain mobile on the surface. On the surface, they wander around until latching into a little nook. (In crystal-growth theory, these 'nooks' are actually tiny kinks in a surface step.) The region just over the edge has plenty of nooks, as it is rounded. So, the edge grows fast and starts to protrude and overhang (stage D). Overhangs from the top and side faces merge in stage E, creating the pocket. Afterwards, water molecules in the pocket surface move about making a more round, disk shape.
About being inexplicable by the standard theory of snow-crystal growth, this standard theory does not include the wandering of the mobile surface molecules over the edge. Now, if the new theory applied only to this corner-pocket formation, then it would be of little importance. But the wandering process cannot apply to only this situation (how would the molecules know when to start or stop?): It must ALWAYS be happening. And, as I mentioned earlier, I realized that the wandering process may help explain many other curious snow-crystal features that I had previously found inexplicable. In other words, it may be a fairly important discovery.
-- JN