| « Black Ice | Caution! » |
Finally got it!
For a few years now, myself and another ice-researcher have been trying to get a research grant from the National Science Foundation to study snow. Well, we just recently heard that the funding came through and I’ll be starting on the project in March. Yeah!
You might wonder what it is that we don’t already know about snow. Well, the better question is, what do we really know? The answer is “not much”. There’s been a lot of experiments in the lab, spanning nearly 100 years now, with many interesting results, but nothing really clear has emerged. Here’s one of the basic problems: In a typical cloud consisting of supercooled droplets and snow crystals, we can calculate the crystal’s rate of growth fairly accurately if we know the crystal shape. But it is extremely difficult to predict the crystal’s shape, and the rate of growth can change a lot with the shape. That’s the thing about snow crystals - they come in a bewildering variety of shapes, and we still have no clue as to why. And if the crystal isn’t surrounded by supercooled droplets, the situation is even worse.
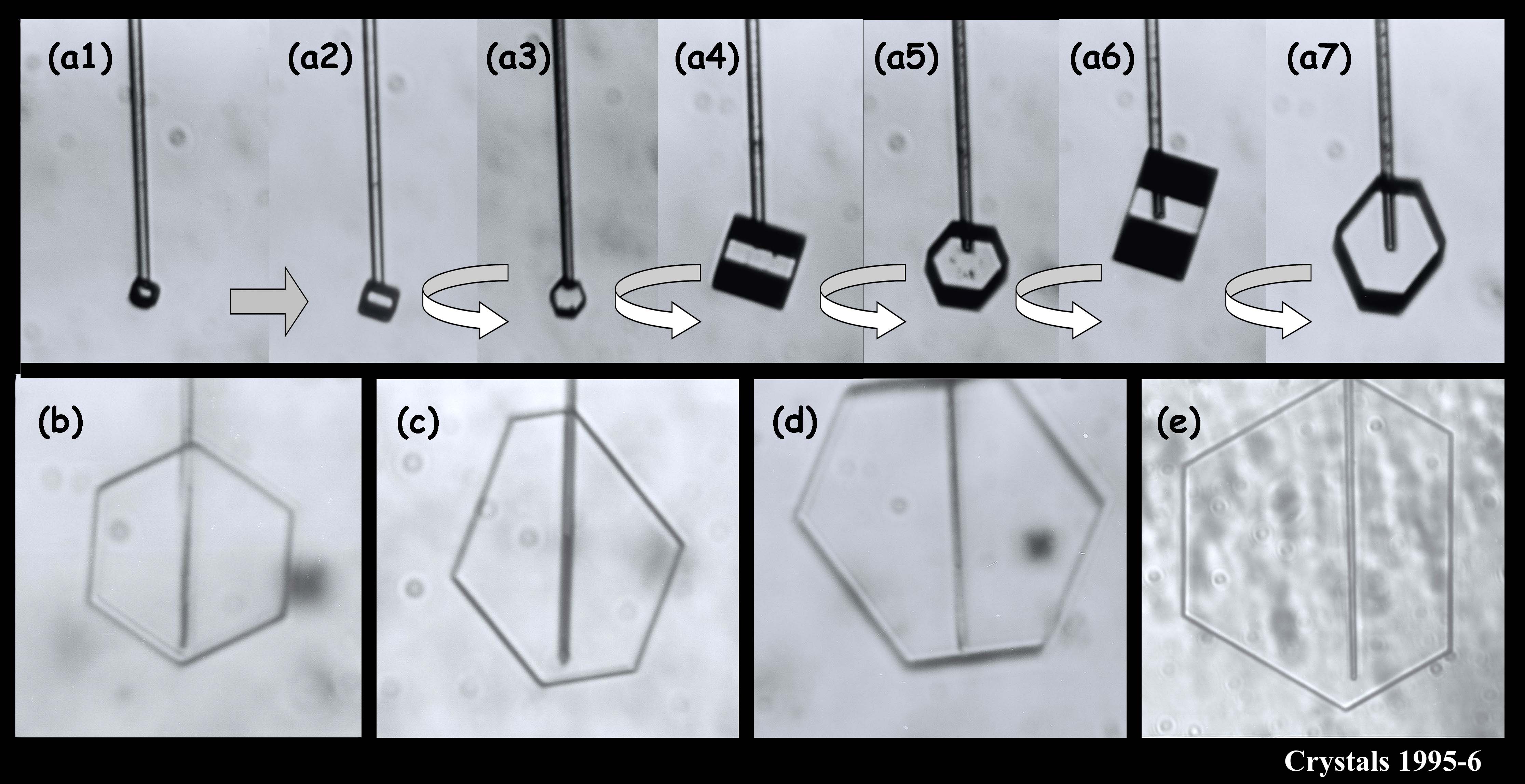
We argued in our proposal that the main reason we don’t have a clear picture about the development of snow crystal shape (and even growth rate) is because of problems with the experimental methods. Instrumental influences easily creep into the experiment and alter the crystal shape. We suggested a new method based on one I helped developed in 1994-1996. In that method, we grew single ice crystals from the tip of an ultrafine glass capillary (about 10x thinner than the typical hair on a person’s head). Some of the images below show what I mean about shape variety.
The crystals are all very small and compact because we were interested in growth with very low humidity. In the series (a1) - (a7), you see the crystal growing as I rotated the capillary to see all of the crystal faces. Sometimes I let a crystal grow for a week or more.
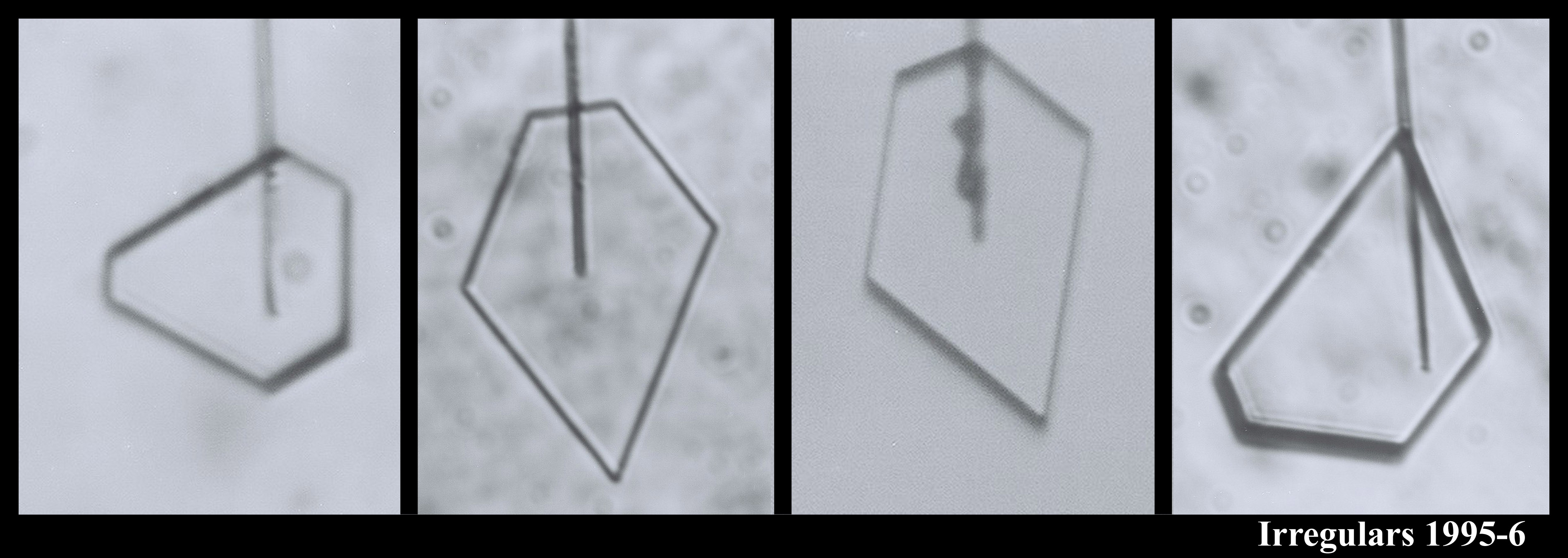
You can see that sometimes a crystal is 5-sided. But the angles between the prismatic faces are always multiples of 60 degrees. I don't think I'll ever see a 5-sided snow crystal that looks exactly like a pentagon.
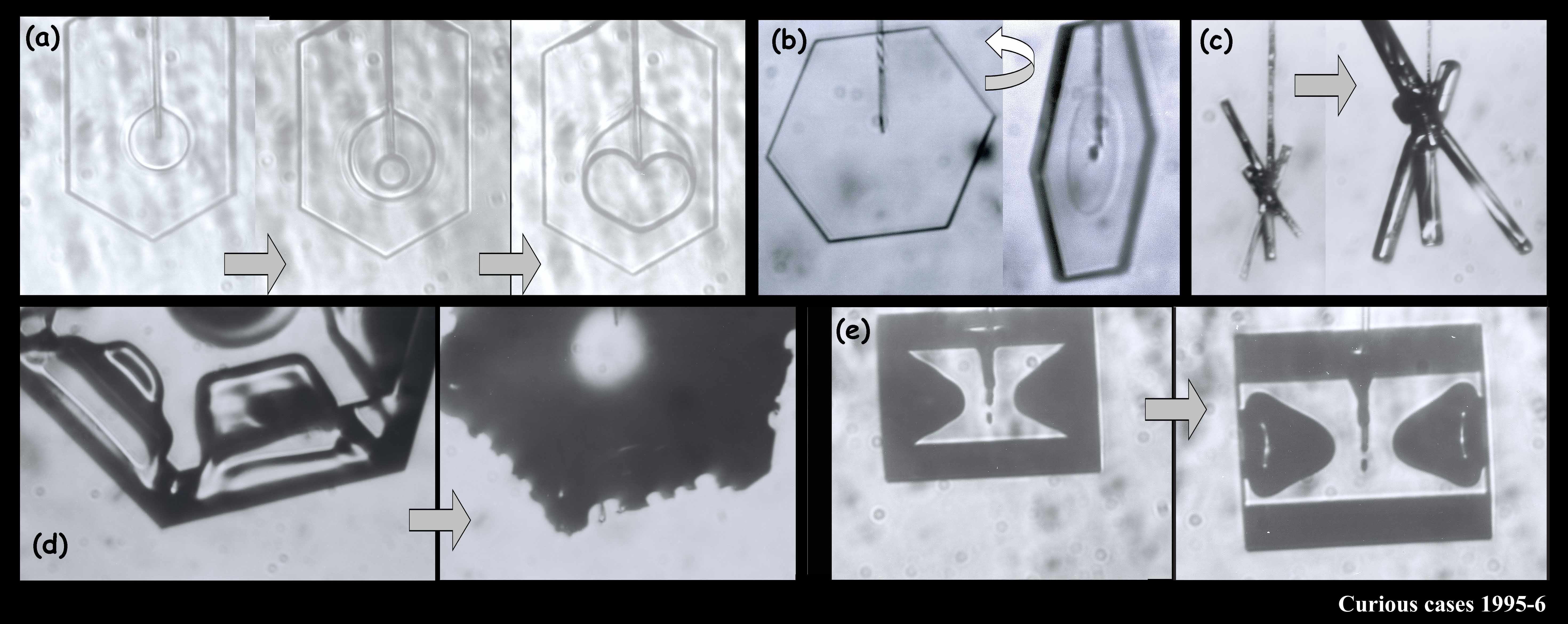
The large crystal at the bottom left & middle was starting to melt. The melting formed cusps on the edges of the face. The one at the top right is called a bullet rosette. These crystals are very common in cirrus clouds and other very low-temperature clouds. The sequence at the upper left shows something we can do with a capillary: we can suck the crystal out from the inside. In that case, I put a vacuum on the other end of the capillary and a void started to form. Then, inexplicably, a crystal started growing inside the void! You never know what will happen in these experiments.
By this time next year, I hope to be able to show new crystals we grew. And sometime soon after that, have some reliable results.
-- Jon