| « Some "Inexplicable" Snow-crystal Features: Applications of Lateral Growth | Martini Hoar (raise a tiny glass?) » |
The Growing Icicle's Hollow Tip
If you inspect the tip of a growing icicle, you might be surprised to find it hollow. Skeptical? Well, if you think the conditions suitable for icicles outside, put a toothpick in your pocket and go outside. Then when you see a likely candidate, poke its tip with the toothpick.
Click on the image to enlarge.
Not all icicles are growing, of course. If the supply of flowing water has dried up, the tip may be solid, or if the air has gotten warm, then the tip may be rounded and wet. The pic below shows a longer, but solid-tipped, icicle next to the growing one. Its melt-water source is too low for the tip to grow.
Transitional situations occur as well, such as the icicle that just started to melt and still has a hollow tip, or the completely solid icicle that just starts growing again and has yet to develop a discernable hollow. The drip may also be intermittent. You might not find that growing icicle right away, but keep that toothpick handy.
As to why a growing tip is hollow, I won't attempt to prove it here, just try to make it seem plausible. Spend a few moments considering the following two simpler situations that show the basic ideas. You can even apply these ideas to other forms involving freezing water...
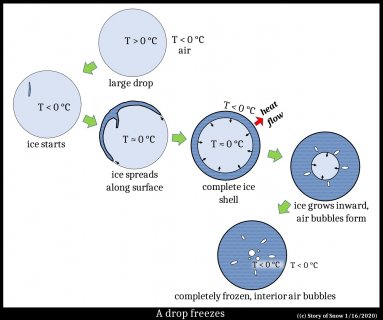
First, consider the freezing drop, surrounded by cold air. The drop cools uniformly because the air is below freezing. At some point ice forms somewhere in the drop.
But turning melt (liquid water) to ice releases heat. This heat warms the drop, but the surrounding air cools the drop (as does evaporation at the surface). So, the surface is coldest, and thus here the ice grows, making an ice shell. See the next two stages in the drawing above. From this shell, the ice grows inward, and once the interior has warmed to 0 C, all excess heat is released outward into the colder air. After all, where else can the heat go?
Also, dissolved air in the melt can collect into small pockets, generally elongated in the direction of freezing as shown in the sketch. In the end, the drop is fully frozen with some air pockets and about the same temperature as the air. Variations to this occur, for example, the shell might retain a little hole as the ice grows inward. Then expansion of the water upon freezing forces some water out the hole, making a little spike. These cases also occur with ice cubes in a tray.
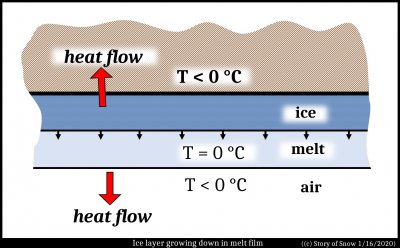
Now consider another case, a horizontal film of water freezes to the underside of a horizontal, sub-zero surface, such as an eave of a roof. The film is thin and flat, and so the ice spreads out in a thin layer against the surface.
As shown in the sketch the remaining melt is warmed to about 0 C, and the heat then flows down to the colder air, as well as upward into the eave. The red arrows show this heat flow, both up into the eave, as well as down into the air below.
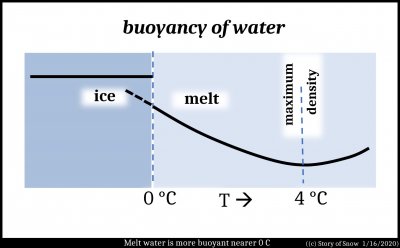
But if the melt is cooling to the colder air below, why doesn't the ice freeze at the bottom? Apparently, this bottom surface does not cool enough to freeze. Several processes are at play here, but one reason that it cannot cool enough to freeze is due to a peculiar property of water: the density maximum near 4 C. Water at this temperature is more dense than warmer water, as expected. That is, a little water at 4 C is less buoyant than water at, say, 6 C, and sinks. But the surprising thing is that water at even lower temperatures, say 1 C, is more buoyant than warmer water at 4 C. (This is why in cold lakes, the water temperature at the bottom will tend to stay near 4 C.) See the plot below.
This trend continues even below 0 C. Such meltwater is called "supercooled". So, any cooling below 0 C at the air-melt surface will produce little pockets of buoyant melt that will rise, pushing warmer 0 C melt down.
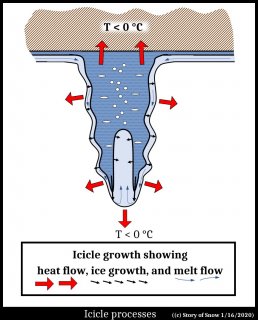
With these two simpler cases, we can see how a hollow tip may be plausible. Initially, the ice may be like the film case just considered. But with more meltwater flowing to the film, the film starts to droop. That is, a drip starts hanging down, like a pendant. The ice shell starts down the side of the pendant drip, like the first case of a drop freezing. But the water at the very tip is warmer, and remains unfrozen as the sides continue freezing downward. The hollow rim has started.
When the icicle has grown down a ways, it may be similar to the sketch below.
The ice is coated with a film of water that slowly flows down to the tip. Along this side, the ice grows outward into the film (like it did in the simple film case), with some heat conducted and convected to the air, and some conducted into the ice (red arrows). Near the tip, the hollow rim also grows inward (releasing heat to the interior), but it grows much faster downward (marked by long black arrows) where there is much more melt to grow into. This discussion really doesn't explain anything, but I hope it makes the shape of the icicle seem more plausible by linking the relevant processes to other phenomena.
Like other common things we take for granted, the icicle has some remarkable aspects. And of course, much more can be discussed about them, such as their strange tendency to ripple. But that's enough for now.
-JN