| « Parting and Bending of Hair Ice | Musings on Bentley’s ‘no two alike’ » |
The Jericho Farmer and the Electric Crystals
[This is the third of the re-posted articles, from 2008.]
For most of his life, and while not attending to farm duties, Wilson A. Bentley was captivated by the beauty of snow crystals. Perhaps then it should not be too surprising that he pondered the physical cause of their symmetry and intricacy. His thoughts turned to electricity as an explanation, perhaps influenced by his era's popular-culture infatuation with the new-fangled electrical devices. Though erring in the details, he was clearly onto something as I will show.
He considered that the crystalline surfaces had electric charges, with more charges concentrated at branch tips (1). When the tips of the branches overflowed with charge, the charges dribbled down the sides to produce ‘growth nuclei’ for the sidebranches. This process, he argued, could explain the symmetry of snow crystals:
"That the crystals, when permitted, attain to such a marvelous degree of symmetry and complicity, shows that the alignment of the growth nuclei, presumably tiny electric charges, is symmetrically regular to an almost unbelievable degree."
Here he connects electricity to the formation of sidebranches and the branch symmetry. In other writings, he connects snow electricity to growth rate, and snow electricity to lightning. In the specific details he was wrong, but in general he was surprisingly prescient. Snow crystals are indeed electric crystals, and the electricity itself is captivating. To see why, consider some of the amazing things that Mr Bentley’s electric crystals can do.
It has been known, at least since Benjamin Franklin's experiments, that lightning in clouds is an electrical discharge. As for the charging, in 1860, William Thomson (later Lord Kelvin) suggested that the falling rain in the thundercloud was the charging current. However, Thomson did not specify how the drops got charged. For a long time, nobody even suspected ice as a player. But in 1904, Mr Bentley asserted that, one, much of the rain in thunderstorms is, in fact, melted snow, and two, the snow is the source of the electricity. Other suggested mechanisms came and went. Yet it wasn’t for another 75 years before experiments and observations made it clear that indeed the electricity in ice is the main source of cloud electricity. Although the details of Bentley’s idea are inconsistent with current knowledge of crystal growth and thunderstorms, we should still give him credit for nominating electric crystals as the key player in thunderstorm electricity.
The current knowledge is this. When left by themselves, ice crystals apparently have negative charges on their surfaces and equal but opposite charges somewhere inside. As Bentley guessed, the amount of charge on the surface seems to increase when the crystals grow, with the greatest charging possibly being on the delicately branched crystals. When these crystals drift upward in a thundercloud, some of them ricochet off their larger, and blobbier, glazed-ice brethren, the hail particles. On average, they shed a little of this surface negative charge in the collision, leaving them with net positive charge and the hail with net negative charge. In each collision, about 10 to 100 thousand negative charges may be transferred. The large, negatively charged hail particles fall lower, and the small, positively charged ice crystals continue to drift upward until they can go no higher (about 6 miles above ground). Here, in the cloud’s upper reaches, a fist-sized volume of air may have tens of thousands of the crystals, so even if each crystal has only a little bit of excess charge, their cumulative charge can be huge. The ice collisions thus separate massive amounts of charge, putting negative charges near cloud bottom and positive charges near cloud top. This is analogous to rubbing a rubber balloon on your head: Each time a hair scrapes against rubber, the hair looses a little negative charge to the rubber. It may not be much, but the cumulative effect of many hairs and many rubs can transfer enough negative charge that, upon removing the balloon, you can hear the crackle of tiny sparks. In the thundercloud, it takes less than 30 minutes for these crystals to accumulate enough charge in the upper reaches for a spark to start. From the initial spark, one or more lightning bolts follow. Mr Bentley’s crystals can put on quite a show!
So, think about these little crystals for a moment – not only do they dazzle us with their beauty when they fall as snow, not only do they shape the land when they stack up in glaciers, but they also create the largest sparks in the world, sparks that have temperatures higher than anywhere else on Earth. Not bad for a tiny bit of frozen water. Not bad at all.
But that is not the only way the ice particles show their electrical nature. The same charge exchange that happens with hail also happens whenever snow blows against something. In the windy plains, snow blowing against wire fences can deposit so much charge that the poor creature that gets too close to the fence may get knocked down by a spark (2). Elsewhere, tent-bound persons in a night-time blizzard have been known to see an electrical glow appear when they place their hand near the tent wall. In this case, each snow crystal that hits the tent charges the fabric a little bit more. The fact that a diffuse glow occurs instead of a spark is likely due to the tent fabric being a poor electrical conductor. This means that the current to the hand is slower and occurs over a broader path – a glow instead of a spark. A similar thing happens when an airplane flies through an icy cloud. The electrical discharge in this case is called St. Elmo’s fire, and it occurs on the outside of the plane. And sometimes, we just hear snow’s electrical effects, as when snow blowing against antennas create radio static. These are just a few of the ways to experience Mr Bentley’s electrical crystal show.
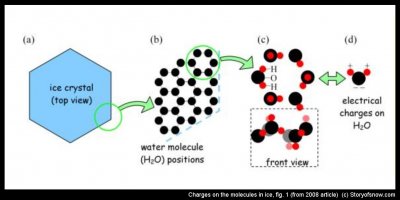
The key process in all of this is the surface charges on the ice. So let’s look a little deeper into the matter and see where these charges come from. If we magnify a region of an ice crystal, such as the top view shown in (a) and (b) above, we see that the positions of the water molecules are nicely arranged in a regular order. This ordering has a honeycomb pattern. But if we look even closer, as in (c), the regularity gets a little messed up. The oxygens (black) in each water molecule sit nice and orderly in a hexagon, but the hydrogens (red) can be arranged many different ways. The only restriction is the Bernal-Fowler bond rule, which says that one hydrogen sits between every pair of adjacent oxygens. But it turns out that the hydrogens are notorious rule breakers, and it is a good thing too, as we will see later.
In many materials, the charges are carried by electrons. But the electrons in the H2O molecule are essentially stuck, unable to leave the molecule. Nevertheless, the hydrogens, which stick out like Mickey Mouse ears, have excess positive charge, whereas Mickey’s ‘chin’ has a corresponding excess of negative charge, as shown in (d) above. This +/– asymmetry makes H2O a polar molecule. Now, there are two ways that the polarity leads to a flow of electrical charge. Consider the charge flow as a dance; the oxygen and hydrogen are the partners, and the partners have two dance steps: the ‘hand-off’ and the ‘twist’.
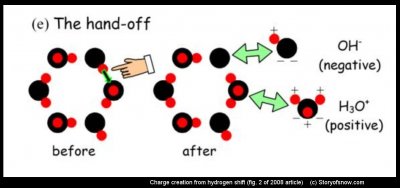
In the hand-off, a hydrogen from one molecule is “handed-off” to the next molecule. If both molecules were originally electrically neutral, as in (e) above, the resulting hand-off (green-black arrow) creates a negative charge (OH-) and a positive charge (H3O+). Subsequently, a hand-off to OH- (or from H3O+) effectively moves the charge through the ice. When two crystals collide, OH- on the surface move from one crystal to another. The reason for the abundance of OH- on the surface is still a mystery, but the twist is probably crucial.
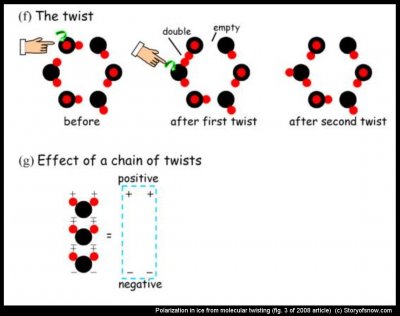
In the twist, a water molecule rotates, effectively removing a hydrogen from one bond and transferring it to another. In the first twist in (f) above, the Bernal-Fowler bond rule gets broken twice: an ‘empty’ bond and a ‘double’ bond are created. In the second twist, the double-bond moves. The empty and double bonds are viewed as two types of charges, but personally, I have trouble viewing them this way. Instead, I think of the charges as arising from a coordinated dance move: When a chain of molecules do the twist, the end result is that the hydrogen positions have become a little more lined up. If we straighten out such a chain, as in (g) above, then the “+” in one Mickey-Mouse ear matches up with a “-” in a chin and the two cancel each other out. Only the “+”s on top and the “–”s on the bottom remain. In reality, the cancellations aren’t perfect, but the effect is the same: the “+”s on top pull on the OH- charges, whereas the “–“s on the bottom push (remember that opposites attract and likes repel). Here is the last important fact: the electrical dance has far more twists than hand-offs – about 10,000 times more. In other words, Bentley’s electric crystals prefer the twist to the hand-off. From what we can tell, all this twisting attracts the OH- to the surface. Once at the surface, these negative charges can move to another surface during a rebounding collision. And that is basically what we now know about the inner workings of the ice-crystal electric show. Enjoy.
In a way, the electricity inside snow crystals is just as captivating as their beauty outside. As we still do not know much about the electrical processes on the inside and the crystallization processes on the outside, the two may someday be found to be closely related, as Wilson Bentley first supposed. It is perhaps not too surprising that he thought about crystal electricity – at that time, electricity was portrayed as a kind of magical fluid. Magazine advertisements boldly asserted the wonders of electrical contraptions like J. Moses’s Electro-Galvanic Spectacles, Heidelberg’s Alternating Current Electric Belt, and the many strange-sounding electrical devices of Dr. Scott. They are all gone now, but we will always have Mr Bentley’s Electric Crystals.
--JN
References and notes
1. This information and the quote are from Bentley’s unpublished notes, discovered and typed up by Duncan Blanchard.
2. R. L. Ives, Weather Phenomena of the Colorado Rockies. J. Franklin Institute 226, 691 (1938).