Parting and Bending of Hair Ice
February 22nd, 2022Hair ice often shows a clear "parting".
By part, I refer to that line along the underlying log surface, either side of which the hair tilts further to the side, analogous to the part one makes in one's own hair with a comb. An explanation for the cause of the part is unlikely to be found in the nature of the pores (from which the ice originates), but rather in the differing rates of growth of adjacent hairs.
The Jericho Farmer and the Electric Crystals
February 22nd, 2022[This is the third of the re-posted articles, from 2008.]
For most of his life, and while not attending to farm duties, Wilson A. Bentley was captivated by the beauty of snow crystals. Perhaps then it should not be too surprising that he pondered the physical cause of their symmetry and intricacy. His thoughts turned to electricity as an explanation, perhaps influenced by his era's popular-culture infatuation with the new-fangled electrical devices. Though erring in the details, he was clearly onto something as I will show.
He considered that the crystalline surfaces had electric charges, with more charges concentrated at branch tips (1). When the tips of the branches overflowed with charge, the charges dribbled down the sides to produce ‘growth nuclei’ for the sidebranches. This process, he argued, could explain the symmetry of snow crystals:
"That the crystals, when permitted, attain to such a marvelous degree of symmetry and complicity, shows that the alignment of the growth nuclei, presumably tiny electric charges, is symmetrically regular to an almost unbelievable degree."
Here he connects electricity to the formation of sidebranches and the branch symmetry. In other writings, he connects snow electricity to growth rate, and snow electricity to lightning. In the specific details he was wrong, but in general he was surprisingly prescient. Snow crystals are indeed electric crystals, and the electricity itself is captivating. To see why, consider some of the amazing things that Mr Bentley’s electric crystals can do.
Musings on Bentley’s ‘no two alike’
February 22nd, 2022[This is the second of the re-posted articles, from 2007.]
Wilson Bentley is famous for his phrase ’no two alike’, but what did he really mean by it? Excerpts in Duncan Blanchard’s book suggest that Bentley was usually referring to only the crystals he photographed (1).
Sometimes though, Bentley seems to be referring to all snow crystals. Maybe sometimes he meant it one way and sometimes the other; however, I wonder if he also had a third, and more profound, meaning in mind, a meaning suggested in his passage (2):
The deeper one enters into the study of Nature, the
further one ventures into and along the by-paths that,
like a mystic maze, thread Nature’s realm in every
direction, the broader and grander becomes the vista
opened up to the view.
If Nature becomes ‘broader and grander’ the deeper one looks, as he so eloquently stated, then of course every snow crystal will be unique; indeed, so too will everything else in Nature. In this meaning, ‘no two alike’ is a very condensed way of saying that Nature will always show you something new. Bentley arrived at this opinion by observing various forms of water, but he applies the idea to all of Nature. This third meaning of ‘no two alike’ reminds me of Kamo no Chomei’s opening line of his early 13th century classic of Japanese literature (3): “The river flows on unceasingly, yet the water is never the same.” Both phrases, Chomei’s and Bentley’s ‘no two alike’, can be interpreted (4) as meaning that the closer one views Nature, the more details one sees. Regardless of Bentley’s intended meaning, I prefer to think that he had this deeper interpretation in mind.
The Snowflake’s Closest of Kin
February 21st, 2022[From 2006 through 2012, I contributed annual articles to the annual newsletter "Snow Crystals" for the Wilson Bentley Historical Society. That newsletter is no longer available, so I will repost my articles here, starting with this one from 2006.]
Wilson Bentley is well known to readers here for his photomicrographs of snow crystals. Snow, however, was only one of the many ‘water wonders’ that held his fascination (ref. 1). Some of these wonders were made of liquid water, such as dew, and some, like the snow crystal, were frozen water (ice).
The frozen type he called “The snowflake’s closest of kin”, and they included hoarfrost, rime, windowpane frost, and ice flowers (2). To obtain photographs of any of them with the quality obtained by Bentley is difficult even now, which is yet another reason to admire Bentley’s skill and perseverance.
On the inside, these ‘kin’ all have the same crystal structure. But they appear different on the outside, largely due to the different ways the water in the surroundings gets to the ice surface. There are many distinct kin because the surrounding water can be in various states (i.e., ice, liquid, and vapor) and there are many ways that each state of water can get to the ice surface. I’ll focus here on snow crystals, hoarfrost, rime, windowpane frost, and ice flowers. These forms are commonly seen by many of us, and have been observed by people for a very long time. So it is easy to think, as I probably once did, that they are well understood by science. But this view is quite mistaken. Yes, we know they all consist of H2O molecules and we know something about the structure of ice, but how exactly they form contain many mysteries. I’ll describe briefly what Bentley thought of them, and what I think is known and not known about them.
Hole in Cloud
February 20th, 2022A region of thin, high cloud becomes ice, making a hole. That is what happened in the picture below.
See the blue hole over the mountain? It may not be circular, but it clearly is a gap where once had been cloud.
If you look at that cloud, you might not realize that it consists nearly entirely of droplets. How do I know that?
The (colored) corona around the sun at the bottom right is a diffraction effect that occurs in thin regions containing nearly uniform-sized droplets. The angular position of the colored rings is solely dependent on the ratio of wavelength to droplet size. So, by noting the color and the position of the ring, one can deduce the droplet size in that region of cloud. Right now, we don't care so much about the droplet size as the mere fact that they must be droplets.
However, the droplets are almost certainly all well below the freezing temperature (perhaps below -20 C), so if a droplet happens to freeze, the resulting crystal will grow rapidly. Growing rapidly, it will both dry out the neighboring air, which causes the surrounding drops to evaporate, and the crystal falls. It falls below the cloud, into drier air, and then it too evaporates (actually, we use the term 'sublimate' for solids, but the process is the same).
If you look near the top of the blue hole, you can see a thin wisp of cloud. That wisp consists of falling ice.
So, that explains why there is a hole, but we have to wonder--why did it happen there?
Well, it must have gotten just a little too cold there, perhaps below -30 C. The cloud layer was probably rising as it moved from right-side to left-side (west-to-east), and a wave was produced where the air went over the mountain ridge that you see. The extra rise in that location brought the droplets into higher, and colder, air where they quickly froze. The wave is invisible, but its effect is not.
--JN
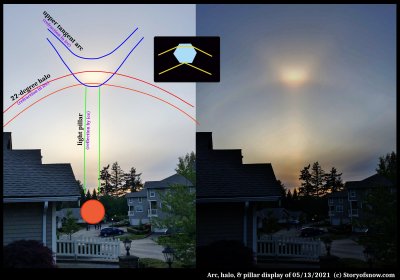
Bright Tangent Arc After Sundown
February 20th, 2022Walking the dog in the evening last May, I saw this bright spot in the sky. The sun had just set and quite a few folks had also noticed it.
I had never seen an optical display after sundown, yet could never think of a reason for this. Indeed, there is no physical reason that some ice-crystal reflection and refraction effects should not be visible after sundown. So, I had simply decided my lack of seeing them was simply due to not having the sun as a good reference point to locate the relatively subtle displays.
This case was not subtle. Indeed, as you can see by the people in the picture, even the "ice-display" novices had noticed.
I will keep this brief, but here is a very crude description of what is happening. The image at the right half is with the contrast cranked up high to help make it clearer.
--JN
Odd Radius Haloes and Pyramidal Crystal Faces
February 19th, 2022Out walking one day last spring, I sensed a subdued illumination--like a thin veil of cloud had drifted in front of the sun. I looked up, and indeed found that there was. Blocking out the sun with my hand, I surveyed the thin veil and saw a strange sight: instead of the usual 22-degree halo around the sun, I saw what looked like two closely spaced haloes near the usual 22-degree spot. I took a picture:
Below, I mark the positions of the observed haloes in green and in black the position that the common 22-degree halo should be.
Capturing Falling Snow in a Cold Fluid
February 15th, 2021Snow is usually imaged in air, the single crystals laying flat on some substrate such as glass. The method is relatively simple, but one must work fast to image the crystal before it appreciably sublimates. Sublimation first rounds out the sharp edges and then causes the crystal to shrink. Generally, this sublimation happens because the photographer is radiating too much heat to the crystal. Conversely, particularly in very cold conditions, the photographer’s breath may deposit fog near the crystal, causing the crystal to grow.
Such issues vanish if one instead captures the snow in a cold fluid before taking the image. To work, this fluid should not dissolve the ice, be less dense than ice, be fluid enough to completely spread over the crystal surface, and be transparent. Other than preserving the crystal, the method has several other advantages. For example, in his laboratory experiments in Hokkaido, Japan, Tsuneya Takahashi lets the crystal fall into a cold suspension of two transparent, cold silicone oils. He sets it up so one fluid is denser than ice, one is lighter than ice, so the crystal falls through the light oil and rests on the (transparent) interface between the two fluids.
This method sounds complicated, so why use it? One, as the oils are immiscible with water, they block water molecules from arriving or leaving the crystal surfaces, so the ice crystal shape is preserved precisely for as long as the fluid is below 32 F (0 C). Two, after imaging the crystal, the fluid is warmed above melting such that the crystal melts into a spherical drop from which he can easily measure the volume and thus infer the mass of the original crystal. A third advantage, more difficult to exploit yet sometimes used, is that he can get top and side views of the same crystal. Other researchers in Japan have also used silicone oils to capture ice crystals in the lab, as well as naturally falling crystals, mainly for the first and third reason. They use just one oil type, a lighter oil. Charles Knight at NCAR in Boulder, Colorado had a fourth reason for using a cold fluid: better imaging. That is, one can image greater depth detail because light scattering off the surfaces is greatly reduced, particularly if the fluid is very clear and has an index of refraction close to that of ice. By reducing the scattering, one can see through surfaces to underlying surfaces. He would use gasoline or hexane fluid.
I don’t have a photomicrography setup to take detailed images of snow, and we rarely get snowfall with nice single crystals anyway, but I wondered how well the method might capture falling snowflakes. That is, could I at least see their rough shapes as they fell through the fluid?
Here, we typically get about one light snowfall per winter (2-3"). A relatively large snowfall happened this past weekend, depositing about seven inches. At first, the particles were small, probably highly rimed single crystals or small aggregates. Later, larger snow particles fell, and these particles were clearly snowflakes (i.e., aggregates). In preparation, the previous night I set out two covered wide-mount jars, one with water, the other with Coleman camping fuel (white gas), which has similar properties to gasoline. In the morning, the one with water had frozen, so I knew the other was also below 32 F.
Outdoors, I set the jar on top of my car, put a wooden chopstick in the jar both to focus on (my camera only has autofocus) and to provide a size reference, set a small LED light panel to the side for brighter illumination, and then opened the lid. The flakes fell into the fluid, and fell down to the bottom of the jar. They fell through the fluid slower than they fell through the air, but it was still too fast for me to see how well they were focused. Turned out that they were not very sharply focused, yet one can still see their general shape and fall orientation (below). In general, the flatter the flake, the more it tends to orient broadside to the fall direction.
Obvious improvements would be a better jar, such as one with a flat, smooth glass front, a better camera, and a thicker fluid to slow down the rate of fall.
Such improvements will have to wait at least until next winter.
--JN
A Stroll on a Mildly Frosty Morning
December 23rd, 2020So far this winter, we've had few frost days in the Redmond, WA area. This morning was typical of the half-dozen or so: a very light dusting of hoar crystals on the roofs and grass. One cannot expect much, and yet I am rarely disappointed. Indeed, it is not until I get close to some icy thing do I notice anything interesting. Sometimes, I still don't notice until I've clicked a few closeups and then viewed on a large computer screen. Here's what I found on this morning's stroll:
The frozen puddle showed some curvy meniscus lines and some straight ice blades, which I've discussed before. The film frost is more mysterious still, but the main curvy pattern is due to the freezing of melt (not vapor deposition, though some of this does occur). The hoar frost shows some scroll crystals, which I recently addressed in an article (no mechanism had previously been argued, but we propose an explanation). See the crystals on the leaf at upper right for the best examples of scrolls, though the details are yet a bit too small. The needle ice is the phenomenon responsible for the crunchy dirt and pushes ice up from the bottom. The ground is warmer below the surface, and here the liquid migrates to the ice front, pushing it all skyward.
Anyway, that's it for this post. No detailed explanations of anything. If you would like to see such explanations, click on the appropriate category in the archives at the right side.
And here's hoping you have a nice, frosty Christmas, wherever you are-
--JN
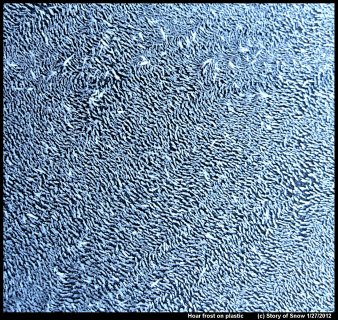
Hoar Frost on Plastic
February 17th, 2020The pattern of hoar frost depends on not just the current and prior conditions, but also on the surface. If the surface is less attractive to water, that is, more hydrophobic, then the hoar crystals tend to be more further spaced apart.
Or even more widely spaced apart. (Click on an image to enlarge it.)
These two images show hoar frost on plastic surfaces (garbage bin lids). If you look closely at the above picture, you will see that the hoar columns are growing off little mounds. These mounds are frozen droplets. You'll also notice that the directions of the ice columns are not random, but instead a given ice column tends to be pointing in nearly the same direction of its neighbors.